2-乙基-己酸稀土盐 | 149-57-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-59 °C
-
沸点:228 °C(lit.)
-
密度:0.906
-
蒸气密度:4.98 (vs air)
-
闪点:230 °F
-
溶解度:1.4g/l
-
暴露限值:ACGIH: TWA 5 mg/m3
-
LogP:2.7 at 25℃
-
物理描述:Ethylhexoic acid is a colorless to light yellow liquid with a mild odor. It will burn though it may take some effort to ignite. It is slightly soluble in water. It is corrosive to metals and tissue. It is used to make paint dryers and plasticizers.
-
颜色/状态:Clear liquid
-
气味:Mild odor
-
蒸汽密度:5.0 (AIR= 1)
-
蒸汽压力:0.03 mm Hg at 20 °C
-
亨利常数:Henry's Law constant = 2.8X10-6 atm-cu m/mol at 25 °C (est)
-
自燃温度:700 °F (371 °C)
-
分解:When heated to decomposition, it emits acrid and irritating fumes.
-
粘度:7.8 mPa s
-
折光率:Index of refraction: 1.4241 at 20 °C/D
-
保留指数:1161 ;1085.19 ;1097 ;1121.3 ;1117
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R63
-
WGK Germany:1
-
海关编码:29159080
-
危险品运输编号:UN 3265 8/PG 2
-
危险类别:6.1
-
RTECS号:MO7700000
-
包装等级:II
-
危险标志:GHS08
-
危险性描述:H361d
-
危险性防范说明:P280
SDS
制备方法与用途
异辛酸可用作油漆和涂料催干剂的中间体、醇酸树脂改性剂,生产过氧化物以作为聚合反应的催化剂以及用于润滑油酯和PVC稳定剂等,市场应用广泛。
制备在2000毫升高压反应釜中依次加入500克异辛醇、258克氢氧化钾及10克甲酸锌。用氮气置换两次以排出系统中的空气后密封,于230℃、2兆帕条件下进行反应。反应6小时后继续保温50分钟,降温至160℃以下,将釜内压力降至常压,加入400毫升蒸馏水搅拌30分钟,得到异辛酸钾水溶液。
将异辛酸钾水溶液转移至烧瓶中,在搅拌下缓慢加入94%的甲酸溶液212克。控制反应温度为60℃,反应30分钟后移至分液漏斗,静置30分钟进行分层分离。水相经简单浓缩可作为商品销售或浓缩成一定浓度(如74%)销售;油相用水洗两次得粗异辛酸,减压蒸馏除去未反应的醇后精馏得异辛酸成品542克。以异辛醇计收率为108.4%,摩尔收率97.92%。
化学性质无色微有臭味的液体。微溶于冷水,溶于热水和乙醚,微溶于乙醇。
用途主要用于制备各种金属盐作为涂料和油漆的催干剂。其酯类可用作增塑剂和羧苄青霉素的原料。2-乙基己酸大部分转化为金属锆、钴、钼、锌等的盐,用于油漆催干剂和聚氯乙烯塑料的热稳定剂;锡盐作为塑料管材的添加剂;钡盐、镉盐用于塑料压延产品和稳定剂。2-乙基已酸及其酯类也用于医药、杀菌剂、金属润滑剂、化妆品等方面,其甘油酯是优良的增塑剂。2-乙基己酸是医药羧苄青霉素的原料,并广泛应用于许多染料、香料的合成。
用途作为油漆和涂料催干剂的中间体,用于醇酸树脂改性剂;生产过氧化物以作为聚合反应(例如PE)的催化剂,用于润滑油酯和PVC稳定剂。此外,还可用作有机合成溶剂。
生产方法国外一些公司用丁醛为原料,经缩合脱水得到2-乙基己烯醛。1. 通过2-乙基己醇氧化法:在氢氧化钠水溶液中以高锰酸钾氧化生成异辛酸钠,再经硫酸中和制得;原料消耗定额为2-乙基已醇1204千克/吨、高锰酸钾3611千克/吨、液碱约1500千克/吨、硫酸约960千克/吨。2. 通过2-乙基己烯醛氧化法:以丙烯羰基合成生产2-乙基己醇的中间产品2-乙基己烯醛为原料,选择性加氢制得2-乙基己醛,再经液相氧化生成2-乙基己醇。3. 通过2-乙基己醇催化脱氢酯化法:在碱性条件下以氧化镉、氧化锌、二氧化锰为催化剂于180至210℃脱氢生成2-乙基己酸酯(异辛酸酯),异辛酸酯经皂化生成相应的盐和醇,盐经硫酸酸化后精馏得异辛酸成品。
类别易燃液体
毒性分级中毒
急性毒性口服 - 大鼠 LD50: 3000毫克/公斤
刺激数据皮肤-兔 450毫克 轻度;眼睛-兔 20毫克 重度
可燃性危险特性遇热、明火易燃;热分解排出辛辣刺激烟雾
储运特性库房通风低温干燥;与氧化剂分开存储
灭火剂 职业标准TWA 4毫克/公斤
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-正己基癸酸 2-hexyldecanoic acid 25354-97-6 C16H32O2 256.429 2-乙基己酸甲酯 methyl 2-ethylhexanoate 816-19-3 C9H18O2 158.241 十六烷 2-乙酸乙酯 hexadecyl 2-ethylhexanoate 59130-69-7 C24H48O2 368.644 异十六烷酸 14-methylpentadecanoic acid 4669-02-7 C16H32O2 256.429 2-乙基已基醛 2-Ethylhexanal 123-05-7 C8H16O 128.214 2-乙基己醇 2-Ethyl-1-hexanol 104-76-7 C8H18O 130.23 2-丁基-2-乙基丙二酸 Ethyl-butyl-malonsaeure 2085-15-6 C9H16O4 188.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— R-(-)-2-ethylhexanoic acid 56006-48-5 C8H16O2 144.214 2-乙基代次羊脂酸 (2S)-ethylhexanoic acid 72377-05-0 C8H16O2 144.214 2-乙基己酸甲酯 methyl 2-ethylhexanoate 816-19-3 C9H18O2 158.241 2-丁基-2-乙基己酸 2-butyl-2-ethylhexanoic acid 32970-62-0 C12H24O2 200.321 3-乙基庚酸 3-ethylheptanoic acid 14272-47-0 C9H18O2 158.241 2-乙基-己酸乙酯 ethyl 2-ethylhexanoate 2983-37-1 C10H20O2 172.268 2-乙基己酸乙烯酯 vinyl 2-ethylhexanoate 94-04-2 C10H18O2 170.252 2-丁基-2-乙基-3-羟基丙酸 (+/-)-2-Ethyl-2-(hydroxymethyl)-hexanoic acid 101051-51-8 C9H18O3 174.24 2-乙基己酸正丁酯 butyl 2-ethylhexanoate 68443-63-0 C12H24O2 200.321 2-乙基己酸己酯 hexyl 2-ethylhexanoate 20748-87-2 C14H28O2 228.375 月桂醇乙基己酸酯 dodecyl 2-ethylhexanoate 56078-38-7 C20H40O2 312.536 十六烷 2-乙酸乙酯 hexadecyl 2-ethylhexanoate 59130-69-7 C24H48O2 368.644 2-乙基己酸辛酯 octyl 2-ethylhexanoate 93777-45-8 C16H32O2 256.429 —— ethylene glycol mono 2-ethylhexanoate 25601-36-9 C10H20O3 188.267 乙基己酸乙基己酯 2-ethylhexyl 2-ethylhexanoate 7425-14-1 C16H32O2 256.429 2-乙基己酸-1-甲基乙酯 isopropyl 2-ethylhexanoate 67024-46-8 C11H22O2 186.294 2-乙基-2-甲基己酸甲酯 methyl 2-ethyl-2-methylhexanoate 3234-80-8 C10H20O2 172.268 2-乙基己酸烯丙酯 allyl 2-ethylhexanoate 58105-49-0 C11H20O2 184.279 2-巯基乙基2-乙基己酸酯 2-Mercaptoethyl 2-ethylhexanoate 67859-57-8 C10H20O2S 204.33 辛基十二醇乙基己酸酯 2-ethaneylhexanoic acid 2-octyldodecanyl ester 69275-04-3 C28H56O2 424.751 2-乙基己醇 2-Ethyl-1-hexanol 104-76-7 C8H18O 130.23 2-乙基已基醛 2-Ethylhexanal 123-05-7 C8H16O 128.214 —— (R)-2-ethylhexanol 50373-29-0 C8H18O 130.23 —— (S)-2-ethylhexan-1-ol 128821-84-1 C8H18O 130.23 2-乙基-2-溴-己酸 2-ethyl-2-bromo-hexanoic acid 35498-12-5 C8H15BrO2 223.11 2-乙基己酸叔丁酯 2-ethylhexanic acid tert-butyl ester 71648-27-6 C12H24O2 200.321 2-乙基己酸酐 2-ethylhexanoic anhydride 36765-89-6 C16H30O3 270.412 —— meso-2-ethylhexanoic acid anhydride 1359865-49-8 C16H30O3 270.412 —— sec-butyl 2-ethylhexanoate —— C12H24O2 200.321 2-乙基己酰基2-乙基过氧己酸酯 2-ethylhexanoic peroxyanhydride 1069-22-3 C16H30O4 286.412 三乙二醇二异辛酸酯 triethylene glycol di-2-ethylhexanoate 94-28-0 C22H42O6 402.572 2-[2-(2-羟基乙氧基)乙氧基]乙基 2-乙基己酸酯 triethylene glycol 2-ethylhexanoate 63468-14-4 C14H28O5 276.373 2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-羟基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙基2-乙基己酸酯 Hexanoic acid, 2-ethyl-, 44-hydroxy-3,6,9,12,15,18,21,24,27,30,33,36,39,42-tetradecaoxatetratetracont-1-yl ester 97862-55-0 C38H76O17 805.011 2-乙基己酸-2-羟基丙酯 2-ethylhexanoic acid 2-hydroxypropyl ester 58921-10-1 C11H22O3 202.294 二乙二醇二(2-乙基己酸)酯 Oxydiethylene bis(2-ethylhexanoate) 72269-52-4 C20H38O5 358.5 正癸酸 1-decanoic acid 334-48-5 C10H20O2 172.268 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:有机合成中的镧系元素。一、二碘化钐碱系统对羧酸的新还原摘要:通过在室温下在质子溶剂的存在下将碱添加到相应的醇中,芳香族和脂肪族羧酸被二碘化钐迅速还原。在 H2O 存在下用二碘化钐同样还原苯甲酸钠,收率良好。在带有羧基、甲酰基、氨基甲酰基、甲氧基和氯基的苯甲酸衍生物的类似反应中,这些官能团也被还原为相应的醇或胺衍生物。有趣的是,苯甲酸的羧基和甲酰基部分被还原为甲基。DOI:10.1246/bcsj.65.3049
-
作为产物:描述:2-乙基己醇 在 13,17-bis(2-methoxycarbonylethyl)-2,7,12,18-tetramethylporphinatocobalt(II) 、 氧气 、 异戊醛 作用下, 以 乙腈 为溶剂, 反应 0.5h, 以34%的产率得到2-乙基-己酸稀土盐参考文献:名称:在异丁醛存在下,使用金属氘代卟啉二甲酯作为催化剂,用双氧将醇快速有氧氧化成羰基化合物摘要:研究了一种简便的仿生方法,使用分子氧作为主要氧化剂,在乙腈中作为反应溶剂,异丁醛作为助催化剂,催化金属氘代卟啉二甲酯 [M(DPDME)] 将醇快速氧化为羰基化合物。在 M(DPDME) 催化剂中,其中 M = Fe(III)、Co(II)、Mn(III)、Ni(II)、Cu(II) 和 Zn(II),发现钴卟啉是最多的活性和有效的催化剂。该催化体系广泛应用于各种醇类的氧化,特别是在温和条件下对芳香醇类的氧化表现出优异的活性。此外,M(DPDME) 是通过一种改进的简便方法通过天然氯化血红素的化学改性制备的,并且已经提出并讨论了醇有氧氧化的替代机制。© 2012 威利期刊公司。杂原子化学 23:295–303, 2012; 在 wileyonlinelibrary.com 上在线查看这篇文章。DOI 10.1002/hc.21017DOI:10.1002/hc.21017
-
作为试剂:描述:对溴乙基苯 在 2-乙基-己酸稀土盐 、 [(2S,2’S)-1,1’-bis((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)-2,2’-bipyrrolidineMnII(OTf)2] 、 双氧水 作用下, 以 乙腈 为溶剂, 反应 3.0h, 以46%的产率得到参考文献:名称:锰配合物催化仲苯甲醇氧化动力学拆分中的不对称自动扩增摘要:在此,手性Mn-氨基吡啶配合物已显示出催化过氧化氢催化烷基芳烃氧化为对映异构体富集的1-芳基链烷醇的能力。所观察到的对映体过量值是由直接对映体选择性苄基CHH羟基化导致的,同时伴随着所得醇的立体收敛氧化动力学拆分。对几种(S,S)-联吡咯烷衍生的Mn配合物进行测试后,发现了一种新型催化剂(6),该催化剂在该系列中显示出最佳的动力学拆分(k rel高达8.8),同时具有足够的反应性和效率(> 1000催化转换)。Mn介导的醇氧化的机理研究证明了亲电活性物质(ρ= -1.2)中,用限速H提取(ķ ħ / ķ d = 2.2),接着氧反弹并且将所得的脱水宝石-二醇以形成酮。有趣的是,尽管要拆分相对较大的1,2-二苯乙醇,k rel在整个反应过程中实际上是恒定的,但对于体积较小的醇,k rel随着转化率的提高而增加,这与1-芳基链烷醇的光学纯度不断提高相一致。后者作为辅助配体参与氧化,有助于手性识DOI:10.1002/cctc.201700438
文献信息
-
[EN] COMPOUNDS<br/>[FR] COMPOSÉS申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2018137593A1公开(公告)日:2018-08-02Provided are novel compounds that inhibit LRRK2 kinase activity, processes for their preparation, compositions containing them and their use in the treatment of or prevention of diseases associated with or characterized by LRRK2 kinase activity, for example Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis (ALS).提供了抑制LRRK2激酶活性的新化合物,以及它们的制备方法、含有它们的组合物以及它们在治疗或预防与LRRK2激酶活性相关或以其为特征的疾病中的用途,例如帕金森病、阿尔茨海默病和肌萎缩侧索硬化症(ALS)。
-
[EN] PHENOTHIAZINE DERIVATIVES AND USES THEREOF<br/>[FR] DÉRIVÉS DE PHÉNOTHIAZINE ET LEURS UTILISATIONS申请人:CAMP4 THERAPEUTICS CORP公开号:WO2019195789A1公开(公告)日:2019-10-10The present invention provides phenothiazine compounds, processes for their preparation, pharmaceutical compositions comprising the compounds, and the use of the compounds or the compositions in the treatment of various diseases or conditions, for example ribosomal disorders and ribosomopathies, e.g. Diamond Blackfan anemia (DBA).
-
Bna Conjugates and Methods of Use申请人:James Kenneth D.公开号:US20080207505A1公开(公告)日:2008-08-28Modified natriuretic compounds and conjugates thereof are disclosed in the present invention. In particular, conjugated forms of hBNP are provided that include at least one modifying moiety attached thereto. The modified natriuretic compound conjugates retain activity for stimulating cGMP production, binding to NPR-A receptor, decreasing arterial blood pressure and in some embodiments an improved half-life in circulation as compared to unmodified counterpart natriuretic compounds. Oral, parenteral, enteral, subcutaneous, pulmonary, and intravenous forms of the compounds and conjugates may be prepared as treatments and/or therapies for heart conditions particularly congestive heart failure. Modifying moieties comprising oligomeric structures having a variety of lengths and configurations are also disclosed. Analogs of the hBNP compound are also disclosed, having an amino acid sequence that is other than the native sequence.
-
Process for preparing polyol esters申请人:Adamzik Michael公开号:US20110087045A1公开(公告)日:2011-04-14The present invention relates to a process for preparing polyol esters by reacting polyols with linear or branched aliphatic monocarboxylic acids having 3 to 20 carbon atoms by partial recycling of the aliphatic monocarboxylic acid removed into the esterification reaction or into subsequent esterification batches.
-
PROCESS FOR PREPARING POLYOL ESTERS申请人:Frey Guido D.公开号:US20120190883A1公开(公告)日:2012-07-26The present invention relates to a process for preparing polyol esters by reacting polyols with linear or branched aliphatic monocarbocxylic acids having 3 to 20 carbon atoms, the reaction taking place in the presence of a Lewis acid comprising at least one element from groups 4 to 14 of the Periodic Table of the Elements as catalyst, and in the presence of an adsorbent, the reaction product being subjected subsequently to a steam treatment.
表征谱图
-
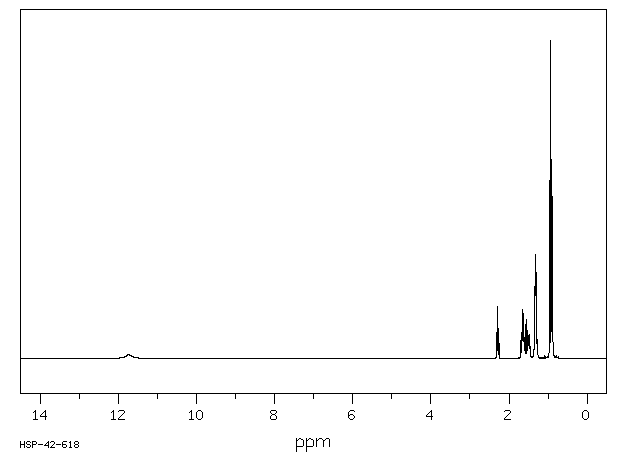
氢谱1HNMR
-
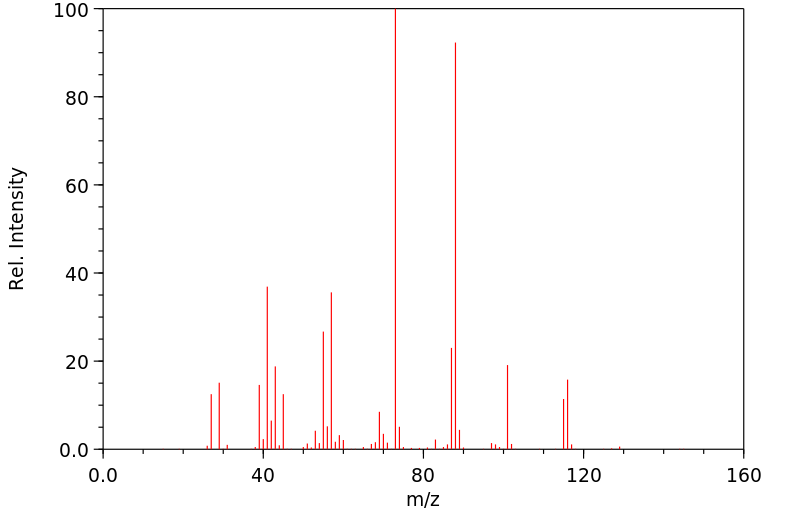
质谱MS
-
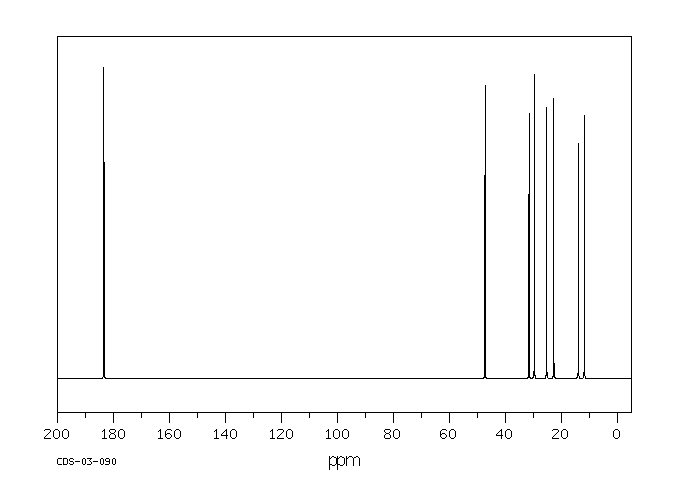
碳谱13CNMR
-
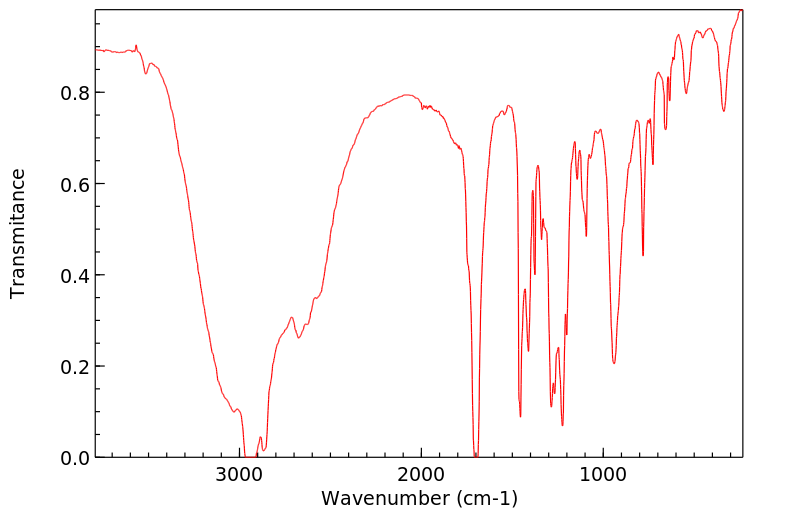
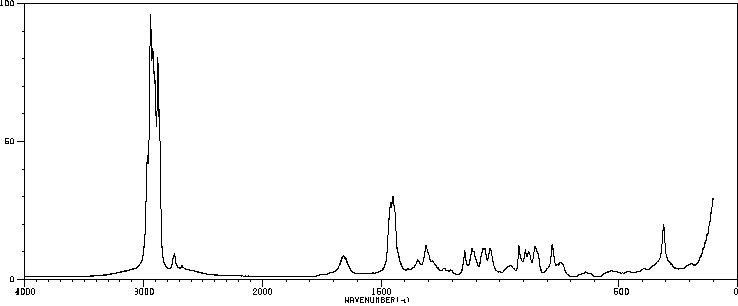
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息