2-苯氧基丙酰氯 | 122-35-0
中文名称
2-苯氧基丙酰氯
中文别名
——
英文名称
2-phenoxypropanoic acid chloride
英文别名
2-phenoxypropionyl chloride;2-phenoxypropanoyl chloride
CAS
122-35-0
化学式
C9H9ClO2
mdl
MFCD00018810
分子量
184.622
InChiKey
BDSSZTXPZHIYHM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:246°C
-
密度:1,18 g/cm3
-
闪点:>100°C
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:8
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R34
-
海关编码:2918990090
-
包装等级:II
-
危险类别:8
-
危险品运输编号:3265
-
储存条件:存放于惰性气体中,并避免接触湿气(以免发生分解)。
SDS
2-Phenoxypropionyl Chloride Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Phenoxypropionyl Chloride
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
2-Phenoxypropionyl Chloride
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Phenoxypropionyl Chloride
Percent: >95.0%(T)
CAS Number: 122-35-0
Chemical Formula: C9H9ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Unsuitable extinguishing Water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
hazards: devices should be prepared in case of a fire. Use spark-proof tools and explosion-
proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
May develop pressure. Open carefully.
Use corrosive resistant equipment.
2-Phenoxypropionyl Chloride
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
106°C/0.7kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.18
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous Decomposes in contact with water and liberates toxic gases.
reactions:
Conditions to avoid: Moisture
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
2-Phenoxypropionyl Chloride
Section 11. TOXICOLOGICAL INFORMATION
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Phenoxypropionyl Chloride
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
2-Phenoxypropionyl Chloride
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Phenoxypropionyl Chloride
Percent: >95.0%(T)
CAS Number: 122-35-0
Chemical Formula: C9H9ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Unsuitable extinguishing Water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
hazards: devices should be prepared in case of a fire. Use spark-proof tools and explosion-
proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
May develop pressure. Open carefully.
Use corrosive resistant equipment.
2-Phenoxypropionyl Chloride
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
106°C/0.7kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.18
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous Decomposes in contact with water and liberates toxic gases.
reactions:
Conditions to avoid: Moisture
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
2-Phenoxypropionyl Chloride
Section 11. TOXICOLOGICAL INFORMATION
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-苯氧基丙酸 2-phenoxypropionic acid 940-31-8 C9H10O3 166.177 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-phenoxypropionyl chloride —— C9H9ClO2 184.622 —— (R)-2-phenoxypropionyl chloride 147851-69-2 C9H9ClO2 184.622 (2S)-2-苯氧基-丙酸 (S)-2-phenoxypropanoic acid 1912-23-8 C9H10O3 166.177 3-苯氧基-2-丁酮 3-phenoxybutan-2-one 6437-85-0 C10H12O2 164.204 —— 2-phenoxypropionamide 13532-52-0 C9H11NO2 165.192 (S)-2-苯氧基丙酸甲酯 S-2-phenoxypropionic acidmethyl ester 115880-48-3 C10H12O3 180.203 2-苯氧基丙酸甲酯 methyl 2-phenoxypropanoate 2065-24-9 C10H12O3 180.203 2-苯氧基丙醇 2-phenoxy-1-propanol 4169-04-4 C9H12O2 152.193 (S)-2-甲基-2-苯氧基甲醇 (S)-2-Methyl-2-phenoxy carbinolcid 87860-35-3 C9H12O2 152.193 —— (R)-2-phenoxypropan-1-ol 64658-22-6 C9H12O2 152.193
反应信息
-
作为反应物:描述:参考文献:名称:Direct Conversion of N-Methoxy-N-methylamides (Weinreb Amides) to Ketones via a Nonclassical Wittig Reaction摘要:N-Methoxy-N-methylamides (Weinreb amides) are converted efficiently into ketones by reaction with alkylidenetriphenylphosphoranes and in situ hydrolysis of the product.DOI:10.1021/ol050337b
-
作为产物:描述:参考文献:名称:Plazek et al., Roczniki Chemii, 1935, vol. 15, p. 365,370摘要:DOI:
文献信息
-
Pyrrolobenzodiazepine pyridine carboxamides and derivatives as follicle-stimulating hormone receptor antagonists申请人:Failli A. Amedeo公开号:US20060287522A1公开(公告)日:2006-12-21This invention provides pyrrolobenzodiazepine pyridine carboxamides selected from those of Formula (1), which act as follicle stimulating hormone receptor antagonists. The invention also provides pharmaceutical compositions and methods of treatment utilizing the compounds of Formulae (1) and (2).
-
FLUORENE-TYPE COMPOUND, PHOTOPOLYMERIZATION INITIATOR COMPRISING SAID FLUORENE-TYPE COMPOUND, AND PHOTOSENSITIVE COMPOSITION CONTAINING SAID PHOTOPOLYMERIZATION INITIATOR申请人:Daito Chemix Corporation公开号:US20150259321A1公开(公告)日:2015-09-17According to an embodiment of the present invention, there is provided a novel fluorene-based compound and a photopolymerization initiator using the fluorene-based compound. The fluorene-based compound according to an embodiment of the present invention is represented by the general formula (1). A photopolymerization initiator having additionally high sensitivity can be provided by using the fluorene-based compound. In addition, a photopolymerization initiator that can impart additionally excellent characteristics can be provided by appropriately selecting, for example, a substituent.
-
[EN] PIPERAZINE, [1,4]DIAZEPANE, [1,4]DIAZOCANE, AND [1,5]DIAZOCANE FUSED IMIDAZO RING COMPOUNDS<br/>[FR] COMPOSES DE CYCLES ACCOLES IMIDAZO DE PIPERAZINE, [1,4]DIAZEPANE, [1,4]DIAZOCANE, ET [1,5]DIAZOCANE申请人:3M INNOVATIVE PROPERTIES CO公开号:WO2005066172A1公开(公告)日:2005-07-21Piperazine, [1,4]diazepane, [1,4]diazocane, and [1,5]diazocane fused imidazo ring compounds (i.e., imidazoquinolines, tetrahydroimidazoquinolines, imidazonaphthyridines, tetrahydroimidazonaphthyridines, and imidazopyridines), pharmaceutical compositions containing the compounds, intermediates, methods of making, and methods of use of these compounds as immunomodulators, for inducing or inhibiting cytokine biosynthesis in animals and in the treatment of diseases including viral and neoplastic diseases are disclosed.
-
Sulfonamido-and amidophenyl N-methylcarbamates and use as insecticides申请人:Mobil Oil Corporation公开号:US04051254A1公开(公告)日:1977-09-27There are provided phenyl N-methylcarbamates substituted in the 4-position with groups, such as alkanesulfamido, haloalkanesulfamido, alkoxyalkanamido, haloalkanamido, alkylthioalkanamido, or furoylamido. These compounds are effective for the control of several insect species as systemic and stomach poison insecticides, as larvicides, and as ovicides.
-
Synthesis and structure–activity relationships of novel arylalkyl 4-benzyl piperazine derivatives as σ site selective ligands作者:S YounesDOI:10.1016/s0223-5234(00)00113-6日期:2000.1Continuing our previous work that established that some chromones substituted by an aryl alkyl piperazino alkyl side chain are potent and selective sigma ligands and could be interesting in the treatment of psychosis, we synthesized 60 new compounds, replacing the chromone moiety by various cyclic systems. Many derivatives bind to the sigma sites in the nanomolar range and are generally selective in comparison
表征谱图
-
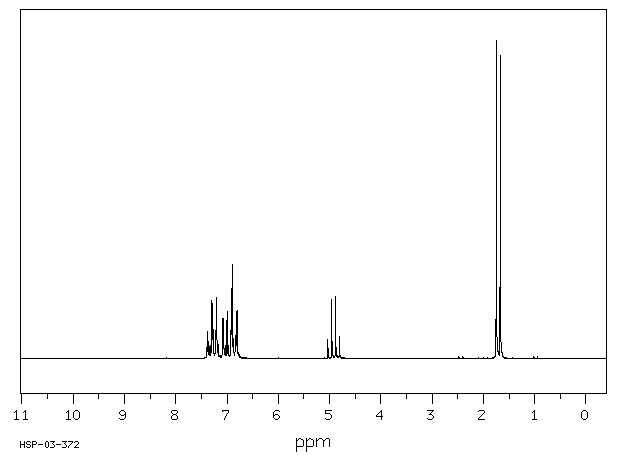
氢谱1HNMR
-
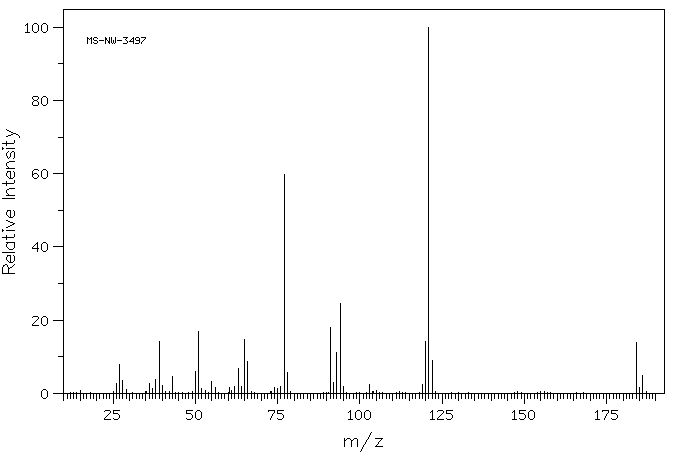
质谱MS
-
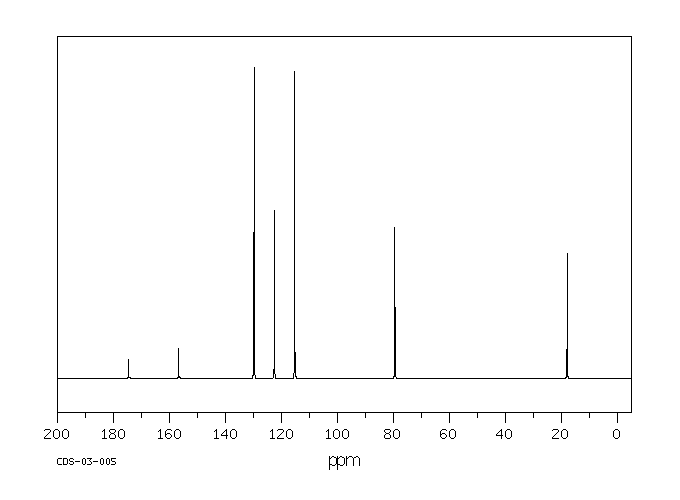
碳谱13CNMR
-
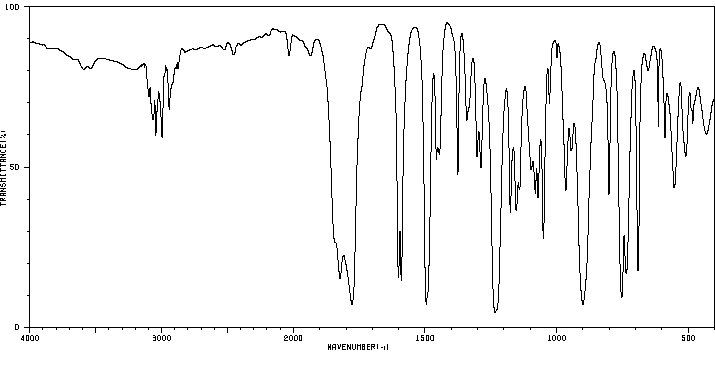
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯