磺胺噻唑 | 72-14-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:200-202 °C (lit.)
-
沸点:479.5±47.0 °C(Predicted)
-
密度:1.4629 (rough estimate)
-
溶解度:水中的溶解度0.5克/升
-
物理描述:Sulfathiazole is a white crystalline powder. Is dimorphous: form I is consists of prismatic rods and form II of six-sided plates and prisms. Insoluble in water and soluble in dil aqueous acid and aqueous base.
-
颜色/状态:Brown plates, rods or powder from 45% alcohol
-
蒸汽压力:4.22X10-8 mm Hg at 25 °C (est)
-
稳定性/保质期:
The stability of sulfamethazine (sulfadimidine) sulfathiazole, sulfameter (sulfamethoxydiazine), and sulfacetamide during acid hydrolysis in one mol/L of hydrochloric acid under increased temperature was examined using high pressure liquid chromatography. Kinetic characteristics of the process of decomposition were calculated from the found values of the concentration of undecomposed sulfonamide in relation to time. The calculated values of activation energy for the individual sulfonamides are given. The paper stresses the illustrativeness of HPLC in the examination of decomposition products.
-
分解:When heated to decomposition it emits very toxic fumes of /nitrogen and sulfur oxides/.
-
解离常数:pKa1 = 2.2; pKa2= 7.24
-
碰撞截面:150.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:122
-
氢给体数:2
-
氢受体数:6
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xi
-
安全说明:S26
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:2935009090
-
包装等级:III
-
危险类别:6.1(b)
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:本品应密封、置于阴凉干燥处并避免光照保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 磺胺噻唑
产品名称
1.2 鉴别的其他方法
4-Amino-N-(2-thiazolyl)benzenesulfonamide
N1-(2-Thiazolyl)sulfanilamide
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 5)
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H303 吞咽可能有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 4-Amino-N-(2-thiazolyl)benzenesulfonamide
别名
N1-(2-Thiazolyl)sulfanilamide
: C9H9N3O2S2
分子式
: 255.32 g/mol
分子量
组分 浓度或浓度范围
Sulfathiazole
<=100%
化学文摘登记号(CAS 72-14-0
No.) 200-771-5
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 200 - 202 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 小鼠 - 4,500 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
致癌性 - 小鼠 - 经口
肿瘤发生:符合RTECS标准的可疑致癌试剂。 肿瘤发生:系统性处理后的肿瘤类型未见自发型。
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
生殖毒性 - 大鼠 - 经口
对生殖的影响:雄性生育力指数(例如#雄性使雌性受精每#雄性与可生育尚未受孕雌性交配)。
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: WP2360000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
磺胺噻唑为白色或淡黄色的结晶粉末;临床上属磺胺类药物,用于治疗肺炎球菌、脑膜炎双球菌、淋球菌和溶血性链球菌的感染。
生物活性Sulfathiazole是一种有机硫化合物,已被用作一种短效的磺胺类药物。
靶点抗菌
体外研究在包含不同废水矩阵的两个间歇式反应器中,Sulfathiazole (20 μg/L) 在其中一个反应器中于第31到38天开始降解。在硝化过程中(S3),Sulfathiazole 的降解速率高于 sulfamethoxazole 或 sulfamethazine。在 pH 9 下,从示踪肥料淤泥样品中恢复的 Sulfathiazole 百分比为64%。Sulfathiazole 的酸度常数 pKa 为7.1,保留时间 tR 为7.8。Sulfathiazole 的 S/N 值在1 mg/kg 水平下高于100。Sulfathiazole 对无机吸附剂的吸附表现出显著的 pH 依赖性,与吸附物形态和吸附剂电荷性质一致。Sulfathiazole 阳离子对吸附到黏土矿物最重要,其次是中性物质。Sulfathiazole 至少具有五种晶体或多晶型:I、II、III、IV 和 V。尽管氨基和亚氨基形态可能存在于 sulfathiazole 分子中,但仅在固态下以亚氨基形式存在。在紫外线B (UV-B) 存在下,Sulfathiazole (94.9 mg/L) 显著增加活性氧(ROS) 的生成和脂质过氧化。暴露于 Sulfathiazole 和 UV-B 光下时,α-酯酶、血红蛋白和卵黄生成素 mRNA 显著上调,并显著影响水蚤的存活。
用途 生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-[4-(1,3-噻唑-2-基氨基磺酰基)苯基]乙酰胺 4'-(thiazol-2-ylsulfamoyl)acetanilide 127-76-4 C11H11N3O3S2 297.359 硝磺胺噻唑 Nisulfazole 473-42-7 C9H7N3O4S2 285.304 —— 2-(p-Carbomethoxyaminobenzenesulfamido)thiazole 33119-99-2 C11H11N3O4S2 313.358 —— 2-sulfanilylamino-thiazole-5-carboxylic acid 5664-51-7 C10H9N3O4S2 299.331 —— N,N-bis(4-acetamidobenzenesulfonyl)-2-aminothiazole —— C19H18N4O6S3 494.573 —— 2-(4-chloro-benzenesulfonylamino)-thiazole-5-carboxylic acid ethyl ester 859486-36-5 C12H11ClN2O4S2 346.815 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(methylamino)-N-(thiazol-2-yl)benzenesulfonamide 70586-66-2 C10H11N3O2S2 269.348 —— 4-hydrazinyl-N-(thiazol-2-yl)benzenesulfonamide 65446-43-7 C9H10N4O2S2 270.336 —— 4-formylamino-N-thiazol-2-yl-benzenesulfonamide 786-25-4 C10H9N3O3S2 283.332 4-叠氮基-N-(噻唑-2-基)苯磺酰胺 4-azido-benzenesulfonic acid thiazol-2-ylamide 36326-87-1 C9H7N5O2S2 281.319 —— 4-isothiocyanato-N-thiazol-2-yl-benzenesulfonamide 51908-30-6 C10H7N3O2S3 297.382 N-[4-(1,3-噻唑-2-基氨基磺酰基)苯基]乙酰胺 4'-(thiazol-2-ylsulfamoyl)acetanilide 127-76-4 C11H11N3O3S2 297.359 —— sulfanilic acid-(5-bromo-thiazol-2-ylamide) 100246-23-9 C9H8BrN3O2S2 334.217 —— N-(thiazol-2-yl)-4-thioureidobenzenesulfonamide —— C10H10N4O2S3 314.413 —— 4-iodo-N-(thiazol-2-yl)benzenesulfonamide 148901-09-1 C9H7IN2O2S2 366.203 —— N-sulfanilyl-sulfanilic acid thiazol-2-ylamide 393124-71-5 C15H14N4O4S3 410.499 —— N-benzenesulfonyl-sulfanilic acid thiazol-2-ylamide —— C15H13N3O4S3 395.484 —— N-propionyl-sulfanilic acid thiazol-2-ylamide 32769-12-3 C12H13N3O3S2 311.386 2-氯-N-{4-[(1,3-噻唑-2-氨基)磺酰基]苯基}乙酰胺 2-chloro-N-(4-(N-(thiazol-2-yl)sulfamoyl)phenyl)acetamide 104246-27-7 C11H10ClN3O3S2 331.804 —— N-mercaptoacetyl-sulfanilic acid thiazol-2-ylamide 29873-34-5 C11H11N3O3S3 329.425 —— N-phenyl-N'-(4-thiazol-2-ylsulfamoyl-phenyl)-urea —— C16H14N4O3S2 374.444 —— N-methyl-N'-(4-thiazol-2-ylsulfamoyl-phenyl)-thiourea —— C11H12N4O2S3 328.44 —— 1-(4-thiazol-2-ylsulfamoyl-phenyl)-biguanide —— C11H13N7O2S2 339.402 —— N-Phenyl-N'-<4-thiazolyl-(2)-aminosulfonyl-phenyl>-thioharnstoff 93020-91-8 C16H14N4O2S3 390.511 —— 4-(benzylideneamino)-N-(thiazol-2-yl)benzene sulfonamide 77218-47-4 C16H13N3O2S2 343.43 —— N,N'-di[4-(N-thiazol-2-yl-sulfonamyl)-phenyl]-sulfamide —— C18H16N6O6S5 572.692 —— 2-cyano-N-(4-(N-(thiazol-2 yl)sulfamoyl)phenyl)acetamide 33987-99-4 C12H10N4O3S2 322.368 —— N-butyryl-sulfanilic acid thiazol-2-ylamide 32769-13-4 C13H15N3O3S2 325.412 —— 3-Chloro-n-[4-(thiazol-2-ylsulfamoyl)-phenyl]-propionamide —— C12H12ClN3O3S2 345.8 —— N-dichloroacetyl-sulfanilic acid thiazol-2-ylamide 32950-57-5 C11H9Cl2N3O3S2 366.249 —— (4-thiazol-2-ylsulfamoyl-phenyl)-carbamic acid ethyl ester 50910-46-8 C12H13N3O4S2 327.385 —— 4-(benzylamino)-N-(thiazol-2-yl)benzenesulfonamide 874505-08-5 C16H15N3O2S2 345.446 —— N-[4-[(2-thiazolylamino)sulfonyl]-phenyl]pentanamide 32769-14-5 C14H17N3O3S2 339.439 —— 4-{[(4-aminophenyl)methyl]amino}-N-(thiazol-2-yl)benzenesulfonamide 1369424-73-6 C16H16N4O2S2 360.461 —— N-trichloroacetyl-sulfanilic acid thiazol-2-ylamide 99973-88-3 C11H8Cl3N3O3S2 400.694 —— N,N'-bis-(4-thiazol-2-ylsulfamoyl-phenyl)-glutaramide —— C23H22N6O6S4 606.728 —— 4-(3-(4-chlorophenyl)ureido)-N-(thiazol-2-yl)benzenesulfonamide —— C16H13ClN4O3S2 408.889 —— N,N'-bis-(4-thiazol-2-ylsulfamoyl-phenyl)-adipamide 122925-07-9 C24H24N6O6S4 620.755 —— N1,N4-bis(4-(N-(thiazol-2-yl)sulfamoyl)phenyl)succinamide 36474-76-7 C22H20N6O6S4 592.701 —— N-stearoyl-sulfanilic acid thiazol-2-ylamide 104158-71-6 C27H43N3O3S2 521.789 —— 4-(2'-Thiazolyl)sulfamyloxanilsaeure 3347-14-6 C11H9N3O5S2 327.342 —— N-palmitoyl-sulfanilic acid thiazol-2-ylamide 104134-71-6 C25H39N3O3S2 493.735 —— N-myristoyl-sulfanilic acid thiazol-2-ylamide 101034-16-6 C23H35N3O3S2 465.681 —— N-lauroyl-sulfanilic acid thiazol-2-ylamide 100411-17-4 C21H31N3O3S2 437.627 —— N-decanoyl-sulfanilic acid thiazol-2-ylamide 99673-95-7 C19H27N3O3S2 409.574 —— 4-(thiazol-2-ylsulfamoyl)-benzenesulfonyl chloride —— C9H7ClN2O4S3 338.817 —— N-(4-thiazol-2-ylsulfamoyl-phenyl)-malonamic acid 39942-17-1 C12H11N3O5S2 341.368 —— N-(thiazol-2-yl)-4-(3-p-tolylthioureido)benzenesulfonamide 93317-79-4 C17H16N4O2S3 404.538 —— N-allyl-N'-(4-thiazol-2-ylsulfamoyl-phenyl)-thiourea 100796-66-5 C13H14N4O2S3 354.478 —— 4-((4-fluorobenzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide —— C16H12FN3O2S2 361.421 —— 4-(3-(4-chlorophenyl)thioureido)-N-(thiazol-2-yl)benzenesulfonamide 454208-28-7 C16H13ClN4O2S3 424.956 琥珀酰磺胺噻唑 succinylsulfathiazole 116-43-8 C13H13N3O5S2 355.395 —— N-(4-thiazol-2-ylsulfamoyl-phenyl)-maleamic acid 515-57-1 C13H11N3O5S2 353.379 马来磺胺噻唑 Maleylsulfathiazole 515-57-1 C13H11N3O5S2 353.379 —— N-(4-thiazol-2-ylsulfamoyl-phenyl)-glutaramic acid 39942-19-3 C14H15N3O5S2 369.422 —— [(4-thiazol-2-ylsulfamoyl-phenylcarbamoyl)-methoxy]-acetic acid 99513-54-9 C13H13N3O6S2 371.395 —— 4-Dithioglykoloyl-benzolsulfonsaeure-(1)- 91768-89-7 C13H13N3O5S3 387.461 —— N-((4-(N-(thiazol-2-yl)sulfamoyl)phenyl)carbamothioyl)acetamide —— C12H12N4O3S3 356.45 —— 4-((4-(dimethylamino)benzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide —— C18H18N4O2S2 386.499 —— N-(N-acetyl-sulfanilyl)-sulfanilic acid thiazol-2-ylamide —— C17H16N4O5S3 452.536 —— 4-(3-hydroxybenzylamino)-N-(thiazol-2-yl)benzene sulfonamide —— C16H15N3O3S2 361.445 —— 4-[(3-hydroxyphenyl)methylideneamino]-N-(1,3-thiazol-2-yl)benzenesulfonamide 318512-19-5 C16H13N3O3S2 359.43 —— 4-((4-methoxybenzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide —— C17H15N3O3S2 373.456 —— N-[4-[(2-thiazolylamino)sulfonyl]phenyl]cyclohexanecarboxamide —— C16H19N3O3S2 365.477 —— 4-((2-hydroxybenzylidene)amino)-N-(1,3-thiazol-2-yl)benzenesulfonamide 7354-98-5 C16H13N3O3S2 359.43 —— N-(4-(N-(thiazol-2-yl)sulfamoyl)phenyl)cinnamamide —— C18H15N3O3S2 385.467 —— (4-thiazol-2-ylsulfamoyl-phenyl)-oxalamic acid ethyl ester 3561-07-7 C13H13N3O5S2 355.395 —— 4-amino-N-methyl-N-(thiazol-2-yl)benzenesulfonamide 51203-19-1 C10H11N3O2S2 269.348 —— N-(4-thiazol-2-ylsulfamoyl-phenyl)-malonamic acid ethyl ester 104427-40-9 C14H15N3O5S2 369.422 —— N-[4-[(2-thiazolylamino)sulfonyl]-phenyl]benzamide 101117-92-4 C16H13N3O3S2 359.43 —— 4-(2-oxo-2-phenylethylamino)-N-(thiazol-2-yl)benzene-sulfonamide 1256846-70-4 C17H15N3O3S2 373.456 —— 4-(2-hydroxybenzylamino)-N-(thiazol-2-yl)benzene sulfonamide 731835-94-2 C16H15N3O3S2 361.445 —— 3-phenyl-N-(4-(N-(thiazol-2-yl)sulfamoyl)phenyl)propanamide —— C18H17N3O3S2 387.483 —— (4-thiazol-2-ylsulfamoyl-phenyl)-arsonic acid 5433-96-5 C9H9AsN2O5S2 364.234 —— N-[4-(thiazol-2-sulfamoyl)phenyl]pyrimidine-2-carboximidamide 1429897-31-3 C14H12N6O2S2 360.42 —— 4-(3-(3,4-dichlorophenyl)ureido)-N-(thiazol-2-yl)benzenesulfonamide 101423-59-0 C16H12Cl2N4O3S2 443.334 —— 4-((4-nitrobenzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide 300727-83-7 C16H12N4O4S2 388.428 —— 4-[(4-nitrophenyl)methylamino]-N-(1,3-thiazol-2-yl)benzenesulfonamide 1429056-07-4 C16H14N4O4S2 390.444 —— 4-[[4-[(4-methoxyphenyl)methylamino]phenyl]methylamino]-N-(1,3-thiazol-2-yl)benzenesulfonamide 1429056-08-5 C24H24N4O3S2 480.612 —— N-[(4-(thiazol-2-yl)-sulfamoylphenyl)]-5-(1,2-dithiolan-3-yl)pentanamide 1085925-72-9 C17H21N3O3S4 443.636 —— 2-(1,3-thiazol-2-ylamino)-N-[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]acetamide 1402580-90-8 C14H13N5O3S3 395.487 —— 4-(3-methoxybenzylamino)-N-(thiazol-2-yl)benzene sulfonamide —— C17H17N3O3S2 375.472 —— 4-([(3-Methoxyphenyl)methylidene]amino)-N-(1,3-thiazol-2-YL)benzenesulfonamide 905006-24-8 C17H15N3O3S2 373.456 —— 2-cyano-3-(4-thiazol-2-ylsulfamoyl-anilino)-acrylic acid ethyl ester 61679-64-9 C15H14N4O4S2 378.433 —— 4-(2,5-dioxo-2H-pyrrol-1(5H)-yl)-N-(thiazol-2-yl)benzenesulfonamide —— C13H9N3O4S2 335.364 —— Acetamide, 2-(4-chlorophenoxy)-N-(4-((2-thiazolylamino)sulfonyl)phenyl)- 58590-35-5 C17H14ClN3O4S2 423.9 —— N,N-succinyl-sulfanilic acid thiazol-2-ylamide 36340-57-5 C13H11N3O4S2 337.38 —— 4-(2-hydroxy-3-methylbenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-19-3 C17H17N3O3S2 375.472 —— 4-(3-(3-chloro-4-methylphenyl)thioureido)-N-(thiazol-2-yl)benzenesulfonamide 1158838-99-3 C17H15ClN4O2S3 438.983 —— 4-(5-amino-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-26-2 C16H16N4O3S2 376.46 —— 4-(2,6-diamino-pyridin-3-ylazo)-N-thiazol-2-yl-benzenesulfonamide 29817-73-0 C14H13N7O2S2 375.435 —— 4-[(2,3-dichlorobenzylidene)amino]-N-(1,3-thiazol-2-yl)benzenesulfonamide 461674-21-5 C16H11Cl2N3O2S2 412.32 —— 4-cyano-N-(4-thiazol-2-ylsulfamoyl-phenyl)-benzamide 93327-29-8 C17H12N4O3S2 384.439 —— 4-(4-chloro-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-24-0 C16H14ClN3O3S2 395.89 —— 4-((3-nitrobenzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide —— C16H12N4O4S2 388.428 —— 4-(2,3-dichlorobenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-14-8 C16H13Cl2N3O2S2 414.336 —— 4-(5-chloro-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 799250-24-1 C16H14ClN3O3S2 395.89 —— 4-((4-oxo-4,5-dihydrothiazol-2-yl)amino)-N-(thiazol-2-yl)benzenesulfonamide —— C12H10N4O3S3 354.434 —— 3-chloro-N-[4-[(2-thiazolylamino)sulfonyl]phenyl]benzamide —— C16H12ClN3O3S2 393.875 —— 4-(3-hydroxy-4-methoxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-12-6 C17H17N3O4S2 391.472 —— 4-(4-bromo-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-18-2 C16H14BrN3O3S2 440.341 —— 4-(3-amino-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-20-6 C16H16N4O3S2 376.46 —— N-(5-bromo-2-hydroxy-benzylidene)-sulfanilic acid thiazol-2-ylamide 132543-09-0 C16H12BrN3O3S2 438.326 —— 4-(2-hydroxy-4-methoxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 684236-02-0 C17H17N3O4S2 391.472 —— 4-(5-fluoro-2-hydroxybenzylamino)-N-(thiazol-2-yl)benzenesulfonamide 1532593-27-3 C16H14FN3O3S2 379.436 —— N-(toluene-4-sulfonyl)-β-alanine 4-thiazol-2-ylsulfamoyl-anilide 63219-24-9 C19H20N4O5S3 480.59 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:磺胺噻唑 在 N-碘代丁二酰亚胺 、 sodium nitrite 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 以86%的产率得到4-iodo-N-(thiazol-2-yl)benzenesulfonamide参考文献:名称:通过亚硝酸钠和N-卤代琥珀酰亚胺将芳胺直接转化为卤代芳烃摘要:描述了一种在室温下在无金属和无酸条件下,通过与亚硝酸钠(NaNO 2)和N-卤代琥珀酰亚胺(NXS)在DMF中反应,将芳基胺转化为芳基卤化物的一锅通用方法。建议与桑德迈尔反应互补的这一新方案涉及原位产生的硝基卤化物引起芳基胺的亚硝化反应,形成重氮中间体,将其卤化以提供芳基卤化物。DOI:10.1002/chem.201803347
-
作为产物:参考文献:名称:作为潜在 BRAFV600E 抑制剂的新型 2-乙酰氨基、6-甲酰胺取代苯并噻唑的设计和合成 – 其抗增殖活性的体外评价摘要:设计并合成了11 种新的取代苯并噻唑衍生物,以提供致癌 BRAFV600E 激酶的有效抑制剂。它们在多种结直肠癌和黑色素瘤细胞系中被生物学评估为潜在的 BRAFV600E 抑制剂、MEK-ERK 通路抑制剂和抗增殖剂。在所有应用的测定中,类似物2-乙酰氨基-N- [3-(吡啶-2-基氨基)丙基]苯并[d]噻唑-6-甲酰胺( 22 )鉴于其在药物开发中的用途提供了有希望的结果。DOI:10.1002/cmdc.202300322
文献信息
-
[EN] ANTIBACTERIAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIBACTÉRIENS申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2019199979A1公开(公告)日:2019-10-17The present application provides compounds of formula: Methods of using these compounds for killing bacterial growth and treating bacterial infections are also provided.本申请提供了以下化合物的公式:还提供了使用这些化合物杀灭细菌生长和治疗细菌感染的方法。
-
New quinoxaline compounds as DPP-4 inhibitors and hypoglycemics: design, synthesis, computational and bio-distribution studies作者:Yasmin M. Syam、Manal M. Anwar、Somaia S. Abd El-Karim、Samia A. Elseginy、Basma M. Essa、Tamer M. SakrDOI:10.1039/d1ra06799k日期:——The current work represents the design and synthetic approaches of a new set of compounds 6-10 bearing the 1,4-dimethyl-2,3-dioxo-1,2,3,4-tetrahydroquinoxaline-6-sulfonamide scaffold. The biological evaluation revealed that most of the new compounds were promising selective dipeptidyl peptidase-IV (DPP-4) inhibitors and in vivo hypoglycemic agents utilizing linagliptin as a standard drug. The acute目前的工作代表了一组新的化合物 6-10 的设计和合成方法,这些化合物带有 1,4-dimethyl-2,3-dioxo-1,2,3,4-tetrahydroquinoxaline-6-sulfonamide 支架。生物学评估表明,大多数新化合物是有希望的选择性二肽基肽酶-IV (DPP-4) 抑制剂和使用利格列汀作为标准药物的体内降血糖剂。急性毒性检查证实了所有化合物的安全性。分子对接研究将化合物 9a、10a、10f、10g 的显着 DPP-4 抑制活性与其在 DPP-4 活性口袋中的良好拟合联系起来。此外,分子动力学研究表明,10a 和 10g 在 DPP-4 的活性位点内均具有稳定性。QSAR研究表明,预测活动之间的差异非常接近实验抑制效果。此外,化合物 10a 和 10g 均符合 Lipinski 规则,表明它们具有有效的口服生物利用度。化合物 10a 被放射性标记,形成 131I-SQ
-
Design, synthesis and SAR of new-di-substituted pyridopyrimidines as ATP-competitive dual PI3Kα/mTOR inhibitors作者:Aisha A.K. Al-Ashmawy、Fatma A. Ragab、Khaled M. Elokely、Manal M. Anwar、Oscar Perez-Leal、Mario C. Rico、John Gordon、Eugeney Bichenkov、George Mateo、Emad M.M. Kassem、Gehan H. Hegazy、Magid Abou-Gharbia、Wayne ChildersDOI:10.1016/j.bmcl.2017.05.044日期:2017.7PI3Kα/mTOR ATP-competitive inhibitors are considered as one of the promising molecularly targeted cancer therapeutics. Based on lead compound A from the literature, two similar series of 2-substituted-4-morpholino-pyrido[3,2-d]pyrimidine and pyrido[2,3-d]pyrimidine analogs were designed and synthesized as PI3Kα/mTOR dual inhibitors. Interestingly, most of the series gave excellent inhibition for bothPI3Kα/ mTOR ATP竞争性抑制剂被认为是有前途的分子靶向癌症治疗剂之一。基于文献中的铅化合物A,设计并合成了两个相似的2-取代-4-吗啉代-吡啶并[3,2- d ]嘧啶和吡啶并[2,3- d ]嘧啶类似物系列,并将其合成为PI3Kα/ mTOR dual抑制剂。有趣的是,大多数系列均对两种酶均具有优异的抑制作用,IC 50值范围从一位到两位数nM。与许多PI3Kα/ mTOR双重抑制剂不同,我们的化合物显示出对PI3Kα的选择性。基于其强大的酶抑制活性,对PI3Kα的选择性以及在2D细胞培养活力测定中良好的治疗指数,化合物4h选择在3D培养中评估其针对MCF7乳腺癌细胞的IC 50以及与这两种酶的对接研究。
-
[EN] 3-PHOSPHOGLYCERATE DEHYDROGENASE INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE LA 3-PHOSPHOGLYCÉRATE DÉSHYDROGÉNASE ET LEURS UTILISATIONS申请人:RAZE THERAPEUTICS INC公开号:WO2017156181A1公开(公告)日:2017-09-14The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用这些化合物的方法。
-
Kappa agonist compounds and pharmaceutical formulations thereof申请人:——公开号:US20030144272A1公开(公告)日:2003-07-31Compounds having kappa opioid agonist activity, compositions containing them and method of using them as analgesics are provided. The compounds of formulae I, II, IIA, III, IIIA, IIIB, IIIB-i, IV and IVA have the structure: 1 2 wherein R 1 , R 2 , R 3 , R 4 ; and X, X 4 , X 5 , X 7 , X 9 ; Y, Z and n are as described in the specification.提供具有kappa阿片受体激动剂活性的化合物,含有这些化合物的组合物以及使用它们作为镇痛剂的方法。 具有以下结构的化合物I、II、IIA、III、IIIA、IIIB、IIIB-i、IV和IVA: 1 2 其中 R 1 ,R 2 ,R 3 ,R 4 ;和 X,X 4 ,X 5 ,X 7 ,X 9 ; Y,Z和n如规范中所述。
表征谱图
-
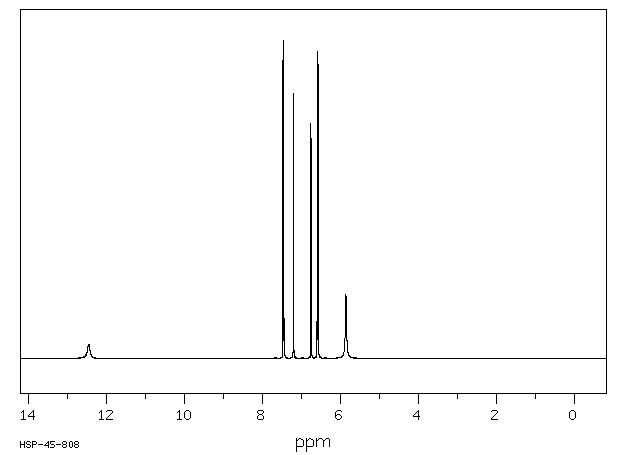
氢谱1HNMR
-
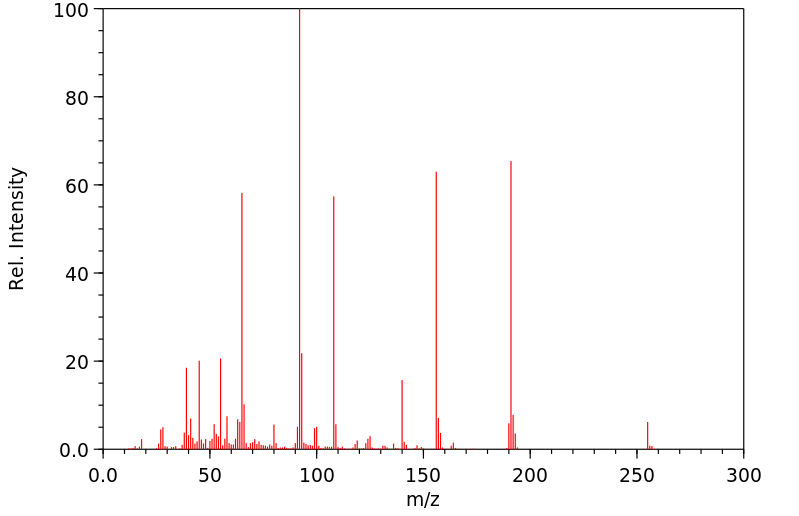
质谱MS
-
碳谱13CNMR
-
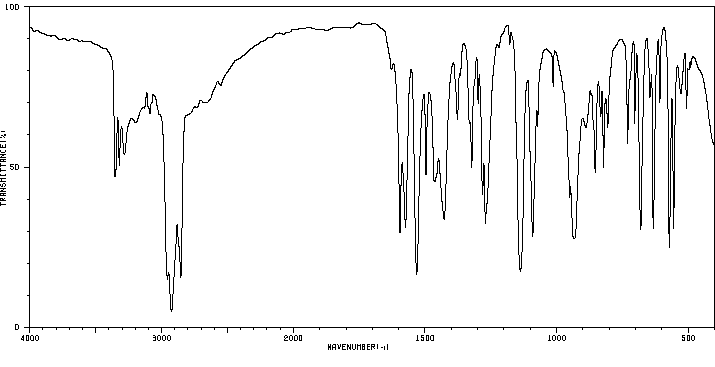
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息