4-苯基-2-吡咯烷酮 | 1198-97-6
中文名称
4-苯基-2-吡咯烷酮
中文别名
4-苯基-2-吡咯酮;苯基-2-吡咯烷酮
英文名称
4-phenylpyrrolidin-2-one
英文别名
4-phenyl-2-pyrrolidinone;4-Phenyl-2-pyrrolidone
CAS
1198-97-6
化学式
C10H11NO
mdl
MFCD01687226
分子量
161.203
InChiKey
HOJZEMQCQRPLQQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:72.0~78.0℃
-
沸点:287.51°C (rough estimate)
-
密度:1.0840 (rough estimate)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2933790090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:存储温度为0-10°C;应存放在惰性气体中;避免接触湿气(可能导致分解),并远离加热源。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Phenyl-2-pyrrolidone
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Phenyl-2-pyrrolidone
CAS number: 1198-97-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H11NO
Molecular weight: 161.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Phenyl-2-pyrrolidone
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Phenyl-2-pyrrolidone
CAS number: 1198-97-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H11NO
Molecular weight: 161.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-tert-butyl-4-phenyl-2-pyrrolidone 138984-08-4 C14H19NO 217.311 4-氨基-3-苯基丁酸盐酸盐 4-amino-3-phenylbutanoic acid 1078-21-3 C10H13NO2 179.219 —— (3R,4S)-2-oxo-4-phenyl-pyrrolidine-3-carboxylic acid 71657-86-8 C11H11NO3 205.213 2-氧代-4-苯吡咯烷-3-羧酸 2-oxo-4-phenylpyrrolidine-3-carboxylic acid 77519-55-2 C11H11NO3 205.213 4-氨基-3-苯基丁酸甲酯 4-amino-3-phenylbutanoic acid methyl ester 84872-79-7 C11H15NO2 193.246 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-4-苯基吡咯烷-2-酮 (4R)-4-phenyl-2-pyrrolidone 71657-88-0 C10H11NO 161.203 —— (S)-4-phenylpyrrolidin-2-one 62624-45-7 C10H11NO 161.203 1-甲基-4-苯基吡咯烷-2-酮 1-methyl-4-phenylpyrrolidin-2-one 54520-84-2 C11H13NO 175.23 —— 1-ethyl-4-phenyl-pyrrolidin-2-one —— C12H15NO 189.257 1-(氯甲基)-4-苯基吡咯烷-2-酮 1-chloromethyl-4-phenyl-2-pyrrolidone 68116-87-0 C11H12ClNO 209.675 —— 1-hydroxymethyl-4-phenyl-pyrrolidin-2-one 68116-73-4 C11H13NO2 191.23 —— 1-(cyanomethyl)-4-phenyl-2-pyrrolidone 126145-48-0 C12H12N2O 200.24 —— 1-acetyl-4-phenylpyrrolid-2-one 55692-55-2 C12H13NO2 203.241 —— 4-Phenyl-1-(4-pyrrolidin-1-ylbut-2-ynyl)pyrrolidin-2-one 137517-99-8 C18H22N2O 282.385 4-苯基-2-吡咯烷酮-1-乙酰胺 carphedon 77472-70-9 C12H14N2O2 218.255 (2-氧代-4-苯基吡咯烷-1-基)乙酸 2-(2-oxo-4-phenylpyrrolidin-1-yl)acetic acid 67118-34-7 C12H13NO3 219.24 —— 1-benzyl-4-phenylpyrrolidin-2-one 108303-98-6 C17H17NO 251.328 —— N-[2-(diethylamino)ethyl]-2-oxo-4-phenyl-1-pyrrolidineacetamide 68644-48-4 C18H27N3O2 317.431 2-氧代-4-苯基-1-吡咯烷乙酸酰肼 2-Oxo-4-phenylpyrrolidine-1-acetic acid hydrazide 77472-71-0 C12H15N3O2 233.27 甲基-2-氧代-4-苯基吡咯烷-1-乙酸酯 methyl-2-oxo-4-phenylpyrrolidine-1-acetate 68497-63-2 C13H15NO3 233.267 4-苯基-1-(三甲基甲硅烷基)-2-吡咯烷酮 1-trimethylsilyl-4-phenyl-2-pyrrolidone 106869-48-1 C13H19NOSi 233.385 N-[3-[双(1-甲基乙基)氨基]丙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺 N-[3-[bis(1-methylethyl)amino]propyl]-2-oxo-4-phenyl-1-pyrrolidineacetamide 68497-64-3 C21H33N3O2 359.512 2-氧代-4-苯基-1-吡咯烷乙酸乙酯 ethyl 2-(2-oxo-4-phenylpyrrolidine-1-yl)acetate 70291-40-6 C14H17NO3 247.294 —— Aminomethyl-2 phenyl-4 pyrrolidine 82256-70-0 C11H16N2 176.261 4-氨基-3-苯基丁酸盐酸盐 4-amino-3-phenylbutanoic acid 1078-21-3 C10H13NO2 179.219 —— N-ethyl-4-(1-methyl-5-oxopyrrolidin-3-yl)benzenesulfonamide —— C13H18N2O3S 282.364 顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺 cis-N-[2-(2,6-dimethyl-1-piperidinyl)ethyl]-2-oxo-4-phenyl-1-pyrrolidineacetamide 88981-74-2 C21H31N3O2 357.496 —— N-isopropyl-4-(1-methyl-5-oxopyrrolidin-3-yl)benzenesulfonamide —— C14H20N2O3S 296.39 —— 4-(1-methyl-5-oxopyrrolidin-3-yl)-N-propylbenzenesulfonamide —— C14H20N2O3S 296.39 —— 1-(1H-imidazol-1-ylmethyl)-4-phenylpyrrolidin-2-one —— C14H15N3O 241.293 —— 1,4-diphenylpyrrolidin-2-one 2889-64-7 C16H15NO 237.301 (+)-(R)-1-叔丁氧羰基-4-苯基-2-吡咯烷酮 tert-butyl 2-oxo-4-phenylpyrrolidine-1-carboxylate 128372-78-1 C15H19NO3 261.321 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:4-苯基吡咯烷酮-2-乙酰胺的合成及解痉活性摘要:众所周知 [i] 各种化合物的解痉特性经常与结构元素的存在有关,这些结构元素包括一个苯基或一个苯环,这些元素相对于一个酰胺基团特别取向,通常是一个片段杂环系统(巴比妥酸、乙内酰脲、吡唑烷二酮、琥珀酰亚胺等)。pyrrolidone-2 的苯基衍生物满足这些要求,事实上,在 5-phenylpyrrolidone-2 的衍生物中发现了具有解痉活性的化合物 [2]。因此,4-苯基吡咯烷酮-2 的衍生物可能显示出明确的解痉作用,正如所指出的,它是苯基替代物 2-吡咯烷酮中在一些其他药理学特性中最活跃的 [3]。DOI:10.1007/bf00765621
-
作为产物:描述:亚苯甲基丙二酸二乙酯 在 palladium on activated charcoal 盐酸 、 氢气 、 三乙胺 作用下, 以 甲醇 、 水 、 甲苯 为溶剂, 60.0 ℃ 、275.79 kPa 条件下, 生成 4-苯基-2-吡咯烷酮参考文献:名称:Inhibition of cyclic adenosine-3',5'-monophosphate phosphodiesterase from vascular smooth muscle by rolipram analogs摘要:Rolipram [(R,S)-4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidone] has been shown to inhibit selectively the cAMP phosphodiesterase (PDE) of vascular smooth muscle. In order to further explore the structural requirements for selective PDE inhibition, we synthesized a series of rolipram derivatives differently substituted either at the pyrrolidinone or at the aromatic ring. Among these compounds, rolipram was the most active compound. Semirigid analogues were prepared and used for an evaluation of the active conformation of rolipram. Structural comparison with two other potent and chemically different smooth muscle cAMP-PDE inhibitors, trequinsin and Ro 20-1724, allows us to propose a first topological model of the smooth muscle cAMP-PDE pharmacophore.DOI:10.1021/jm00127a009
文献信息
-
[EN] 4-HYDROXYPIPERIDINE DERIVATIVES AND THEIR USE AS INHIBITORS OF UBIQUITIN SPECIFIC PROTEASE 19 (USP19)<br/>[FR] DÉRIVÉS DE 4-HYDROXYPIPÉRIDINE ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE LA PROTÉASE 19 SPÉCIFIQUE DE L'UBIQUITINE申请人:ALMAC DISCOVERY LTD公开号:WO2019150119A1公开(公告)日:2019-08-08Inhibitors of ubiquitin specific protease 19 (USP19) of Formula (I) are provided, together with pharmaceutical compositions comprising said inhibitors, and methods of use thereof. The compounds can be used in in the treatment of muscular atrophy, obesity, insulin resistance or type II diabetes or in reducing the loss of muscle mass.
-
GSK-3BETA INHIBITOR申请人:Kori Masakuni公开号:US20110039893A1公开(公告)日:2011-02-17For the purpose of providing a GSK-3β inhibitor containing a 2-aminopyridine compound or a salt thereof or a prodrug thereof useful as an agent for the prophylaxis or treatment of a GSK-3β-related pathology or disease, the present invention provides a GSK-3β inhibitor containing a compound represented by the formula (IA): wherein each symbol is as defined in the specification. or a salt thereof or a prodrug thereof.
-
PROCESS FOR STRAIGHTENING KERATIN FIBRES WITH A HEATING MEANS AND DENATURING AGENTS申请人:Philippe Michel公开号:US20100028280A1公开(公告)日:2010-02-04The invention relates to a process for straightening keratin fibres, comprising: (i) a step in which a straightening composition containing at least two denaturing agents is applied to the keratin fibres, (ii) a step in which the temperature of the keratin fibres is raised, using a heating means, to a temperature of between 110 and 250° C.该发明涉及一种直发角蛋白纤维的拉直过程,包括:(i)将至少两种变性剂含有的拉直组合物涂抹到角蛋白纤维上的步骤,(ii)使用加热装置将角蛋白纤维的温度升高至110至250°C的步骤。
-
[EN] HYDROXYETHYLAMINE DERIVATIVES FOR THE TREATMENT OF ALZHEIMER'S DISEASE<br/>[FR] DERIVES D'HYDROXYETHYLAMINE UTILISES POUR LE TRAITEMENT DE LA MALADIE D'ALZHEIMER申请人:GLAXO GROUP LTD公开号:WO2004050619A1公开(公告)日:2004-06-17The present invention relates to novel hydroxyethylamine compounds of formula (I): (I) having Asp2 (-secretase, BACE1 or Memapsin) inhibitory activity, processes for their preparation, to compositions containing them and to their use in the treatment of diseases characterised by elevated - amyloid levels or -amyloid deposits, particularly Alzheimer's disease.
-
A phase-switch purification approach for the expedient removal of tagged reagents and scavengers following their application in organic synthesis作者:Jason Siu、Ian R. Baxendale、Russell A. Lewthwaite、Steven V. LeyDOI:10.1039/b503778f日期:——In this paper we wish to report on a variety of expedient chemical transformations and purifications achieved via a generic ‘catch and release’ methodology, based on a synthetically inert bipyridyl chelating tag that can be selectively captured with a resin-bound copper(II) species. Utilising this approach we are able to derive many of the same benefits associated with both solid phase synthesis and supported reagent methods.
表征谱图
-
氢谱1HNMR
-
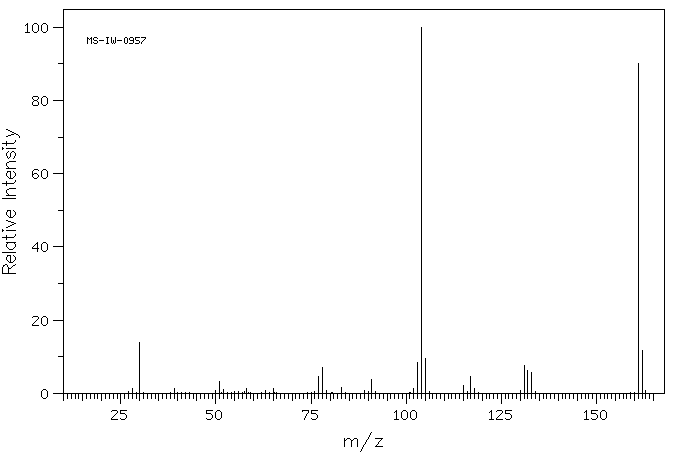
质谱MS
-
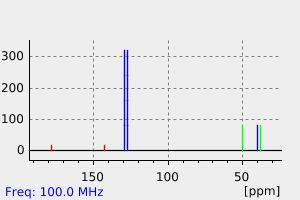
碳谱13CNMR
-
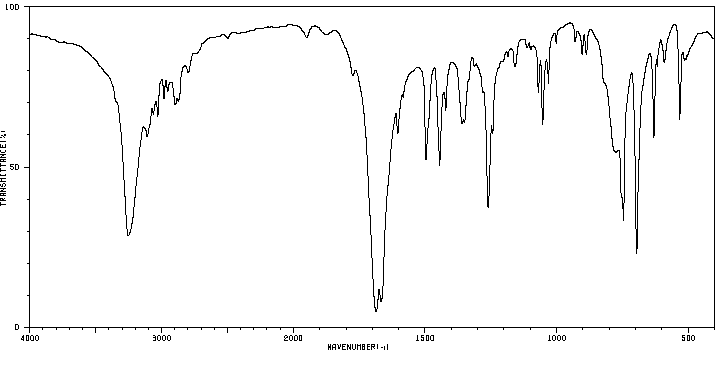
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(2R,2''R)-(-)-2,2''-联吡咯烷
麦角甾-7,22-二烯-3-基亚油酸酯
马来酰亚胺霉素
马来酰亚胺基酰肼盐酸盐
马来酰亚胺基甲基-3-马来酰亚胺基丙酸酯
马来酰亚胺丙酰基-dPEG4-NHS
马来酰亚胺-酰胺-PEG6-琥珀酰亚胺酯
马来酰亚胺-酰胺-PEG6-丙酸
马来酰亚胺-酰胺-PEG24-丙酸
马来酰亚胺-酰胺-PEG12-丙酸
马来酰亚胺-四聚乙二醇-羧酸
马来酰亚胺-四聚乙二醇-丙酸叔丁酯
马来酰亚胺-四聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-六聚乙二醇-羧酸
马来酰亚胺-六聚乙二醇-丙酸叔丁酯
马来酰亚胺-八聚乙二醇-丙酸叔丁酯
马来酰亚胺-二聚乙二醇-丙酸叔丁酯
马来酰亚胺-三(乙烯乙二醇)-丙酸
马来酰亚胺-一聚乙二醇-羧酸
马来酰亚胺-一聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-PEG3-羟基
马来酰亚胺-PEG2-胺三氟醋酸盐
马来酰亚胺-PEG2-琥珀酰亚胺酯
马来酰亚胺
频哪醇硼酸酯
顺式草酸双(-3,8-二氮杂双环[4.2.0]辛烷-8-羧酸叔丁酯)
顺式4-甲基吡咯烷酮-3-醇盐酸盐
顺式4-氟吡咯烷酮-3-醇盐酸盐
顺式3,4-二羟基吡咯烷盐酸盐
顺式3,4-二氨基吡咯烷-1-羧酸叔丁酯
顺式-二甲基 1-苄基吡咯烷-3,4-二羧酸
顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺
顺式-N-Boc-吡咯烷-3,4-二羧酸
顺式-5-苄基-2-叔丁氧羰基六氢吡咯并[3,4-c]吡咯
顺式-5-甲基-1H-六氢吡咯并[3,4-b]吡咯二盐酸盐
顺式-5-氧代六氢环戊二烯并[c]吡咯-2(1H)-羧酸叔丁酯
顺式-5-乙氧羰基-1H-六氢吡咯并[3,4-B]吡咯盐酸盐
顺式-5-(碘甲基)-4-苯基-2-吡咯烷酮
顺式-5-(碘甲基)-4-甲基-2-吡咯烷酮
顺式-4-氧代-六氢-吡咯并[3,4-C]吡咯-2-甲酸叔丁酯
顺式-3-氟-4-羟基吡咯烷-1-羧酸叔丁酯
顺式-3-氟-4-甲基吡咯烷盐酸盐
顺式-2-甲基六氢吡咯并[3,4-c]吡咯
顺式-2,5-二甲基吡咯烷
顺式-1-苄基-3,4-吡咯烷二甲酸二乙酯
顺式-1-甲基六氢吡咯并[3,4-b]吡咯
顺式-(9CI)-3,4-二乙烯-1-(三氟乙酰基)-吡咯烷
顺-八氢环戊[c]吡咯-5-酮盐酸盐
非星匹宁