2-丁基-1H-咪唑-4-甲醛 | 68282-49-5
中文名称
2-丁基-1H-咪唑-4-甲醛
中文别名
2-丁基-1H-咪唑-5-甲醛;2-丁基-咪唑-4-甲醛;2-正丁基-5-甲酰基咪唑;2-正丁基-4-甲酰基咪唑
英文名称
2-n-butyl-1H-imidazole-4-carbaldehyde
英文别名
2-butyl-1H-imidazole-4-carbaldehyde;2-n-butyl-4-imidazolecarboxaldehyde;2-butyl imidazole-4-carboxaldehyde;2-Butyl-4-formyl imidazole;2-n-butylimidazole-4(5)-carbaldehyde;2-n-butyl-4-formylimidazole;2-butyl-1H-imidazole-5-carbaldehyde
CAS
68282-49-5
化学式
C8H12N2O
mdl
——
分子量
152.196
InChiKey
PTHGVOCFAZSNNA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:117-120°C
-
沸点:359.1±15.0 °C(Predicted)
-
密度:1.099±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:11
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:45.8
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2933290090
-
安全说明:S26,S37/39
SDS
| Name: | 2-Butyl-1h-imidazole-4-carbaldehyde 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 68282-49-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 68282-49-5 | 2-Butyl-1H-imidazole-4-carbaldehyde | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 68282-49-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 117 - 120 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H12N2O
Molecular Weight: 152
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 68282-49-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Butyl-1H-imidazole-4-carbaldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 68282-49-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 68282-49-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 68282-49-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 咪唑醛 2-butyl-5-chloro-3H-imidazole-4-carbaldehyde 83857-96-9 C8H11ClN2O 186.641 2-丁基-4-羟甲基咪唑 2-butyl-5-hydroxymethyl-1H-imidazole 68283-19-2 C8H14N2O 154.212 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (9ci)-2-丁基-1H-咪唑-4-羧酸 2-Butyl-5-imidazolecarboxylic acid 473263-84-2 C8H12N2O2 168.195 5-溴-2-丁基-1H-咪唑-4-甲醛 2-n-butyl-4-bromoimidazole-5-carboxaldehyde 139742-78-2 C8H11BrN2O 231.092 咪唑醛 2-butyl-5-chloro-3H-imidazole-4-carbaldehyde 83857-96-9 C8H11ClN2O 186.641 —— 3-(2-butyl-1H-imidazol-4-yl)propan-1-ol —— C10H18N2O 182.266
反应信息
-
作为反应物:描述:参考文献:名称:A New Regioselective Synthesis of 1,2,5-Trisubstituted 1H-Imidazoles and Its Application to the Development of Eprosartan摘要:A new method is presented for the preparation of 1,2-disubstitued-1H-imidazole-5-carboxaldehydes by the reaction of N-monosubstituted amidines with 2-halo-3-alkoxy-2-propenals. The reaction is highly regioselective with ratios of 1,2,5:1,2,4-imidazolecarboxaldehydes ranging from 85:15 to 100: 0. This methodology could be extended with similar results to the synthesis of imidazole-g-nitriles by the reaction of 2-bromo-3-methoxy-2-propenenitrile with N-monosubstituted amidines.DOI:10.1021/jo971304f
-
作为产物:参考文献:名称:Preparation of 2-Substituted 4-Chloro-5-Formylimidazole and 5-Formylimidazole摘要:该发明涉及一种2-取代-5-甲酰基咪唑的制备过程,其中方便地避免了在技术中已知的2-取代-4-羟甲基咪唑的中间高压合成,并且获得了更高的产率。相反,建议通过一锅法合成涉及2-取代-4-氯-5-甲酰基咪唑来制备这种2-取代-5-甲酰基咪唑,从而利用额外的去卤化步骤。此外,发现在制备过程中使用三氟甲磺酸盐催化剂可以显著提高2-取代-4-氯-5-甲酰基咪唑本身的产率和纯度。公开号:US20080200690A1
文献信息
-
Indole- and benzimidazole-substituted imidazole and benzimidazole申请人:E. R. Squibb & Sons, Inc.公开号:US05374615A1公开(公告)日:1994-12-20Novel compounds are disclosed having the formula ##STR1## wherein X, R.sub.1, R.sub.2, R.sub.3, R.sub.4, and R.sub.5 are substituents. These compounds inhibit the action of angiotensin II and are useful, therefore, for example, as antihypertensive agents.揭示了具有以下公式的新化合物##STR1##其中X,R.sub.1,R.sub.2,R.sub.3,R.sub.4和R.sub.5是取代基。这些化合物抑制肾素-血管紧张素II的作用,因此例如作为降压药物是有用的。
-
Imidazolyl-alkenoic acids useful as angiotensin II receptor antagonists申请人:SmithKline Beecham Corporation公开号:US05185351A1公开(公告)日:1993-02-09Angiotensin II receptor antagonists having the formula: ##STR1## which are useful in regulating hypertension and in the treatment of congestive heart failure, renal failure, and glaucoma, pharmaceutical compositions including these antagonists, and methods of using these compounds to produce angiotensin II receptor antagonism in mammals.Angiotensin II受体拮抗剂具有以下结构式:##STR1##,可用于调节高血压,并用于治疗充血性心力衰竭、肾功能衰竭和青光眼,包括这些拮抗剂的药物组合物,以及使用这些化合物在哺乳动物中产生Angiotensin II受体拮抗作用的方法。
-
[EN] 1,2,3,4-TETRAHYDROISOQUINOLINE DERIVATIVES, PREPARATIONS THEREOF AND USES THEREOF<br/>[FR] DERIVES DE 1,2,3,4-TETRAHYDROISOQUINOLINE, LEURS PREPARATIONS ET LEURS UTILISATIONS申请人:ASTRAZENECA AB公开号:WO2005061484A1公开(公告)日:2005-07-07Compounds of general formula (I) wherein D, E, R1, R2, R3, R4, R5, R6 and R7 are as defined in the specification, as well as salts, enantiomers thereof and pharmaceutical compositions including the compounds are prepared. They are useful in therapy, in particular in the management of pain.通式(I)的化合物,其中D、E、R1、R2、R3、R4、R5、R6和R7如规范中所定义,以及盐、对映体以及包括这些化合物的药物组合物已经制备。它们在治疗中很有用,特别是在疼痛管理中。
-
Process for the preparation of formylimidazoles申请人:Lonza AG公开号:US06127548A1公开(公告)日:2000-10-03A process for the preparation of formylimidazoles of the general formula: or its tautomers, in which R.sup.1 is hydrogen or alkyl, and R.sup.2 is hydrogen, halogen or alkyl. In a first stage, an imidazole derivative of the general formula: ##STR1## or its tautomers, in which R.sup.1 and R.sup.2 are as defined above, is converted, by introducing an amino protective group, into an imidazole derivative of the general formula: ##STR2## or its tautomers, in which R.sup.3 is an amino protective group. Such derivative is formylated in a second stage in the presence of an organometallic compound and a suitable electrophile to give an imidazole derivative of the general formula: ##STR3## or its tautomers, in which R.sup.1, R.sup.2 and R.sup.3 are as defined above. Then, in a third stage, the end product of the formula I is obtained by cleaving off the amino protective group.
-
Application of High Throughput Screening to Heterogeneous Liquid and Gas Phase Oxidation Catalysis作者:Anil Guram、Alfred Hagemeyer、Claus G. Lugmair、Howard W. Turner、Anthony F. Volpe、W. Henry Weinberg、Karin YaccatoDOI:10.1002/adsc.200303170日期:2004.2The application of combinatorial methods to oxidation catalysis in the liquid and gas phases is described. New lead materials have been discovered for the selective liquid phase oxidation of alcohols to aldehydes/ketones catalyzed by vanadium supported on carbon, for the low temperature CO oxidation/light off for cold start automotive emissions control over supported noble metals and perovskites, for
表征谱图
-
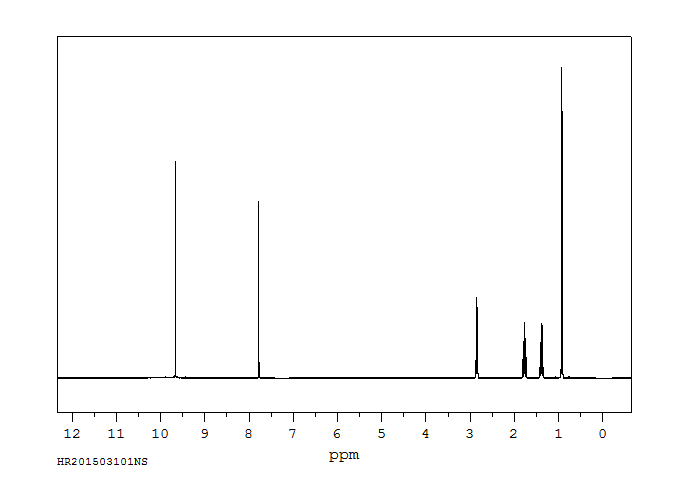
氢谱1HNMR
-
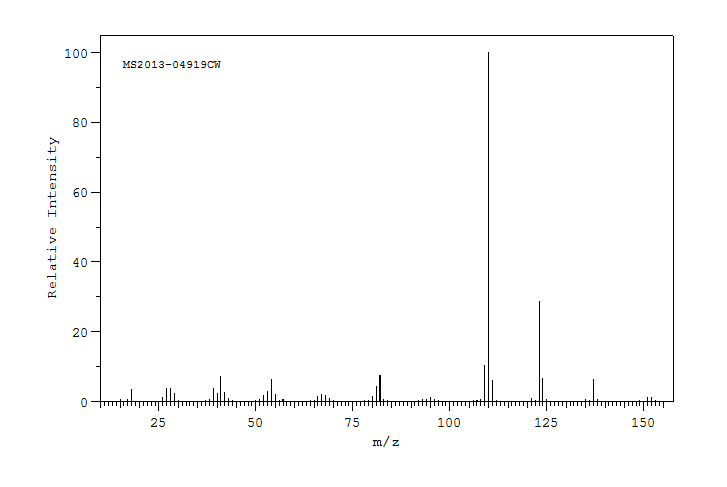
质谱MS
-
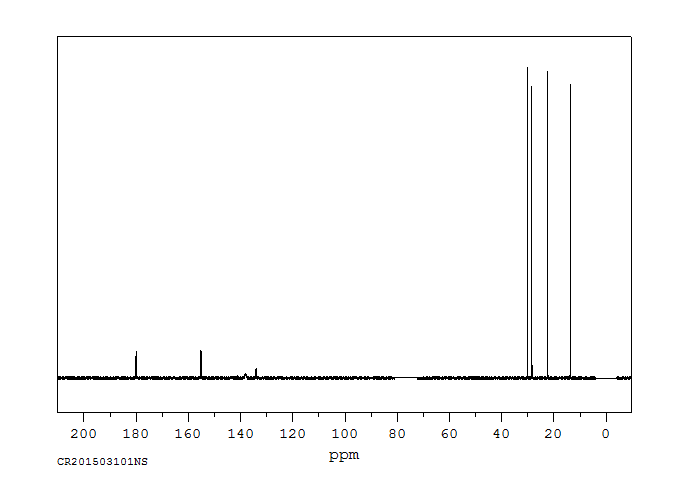
碳谱13CNMR
-
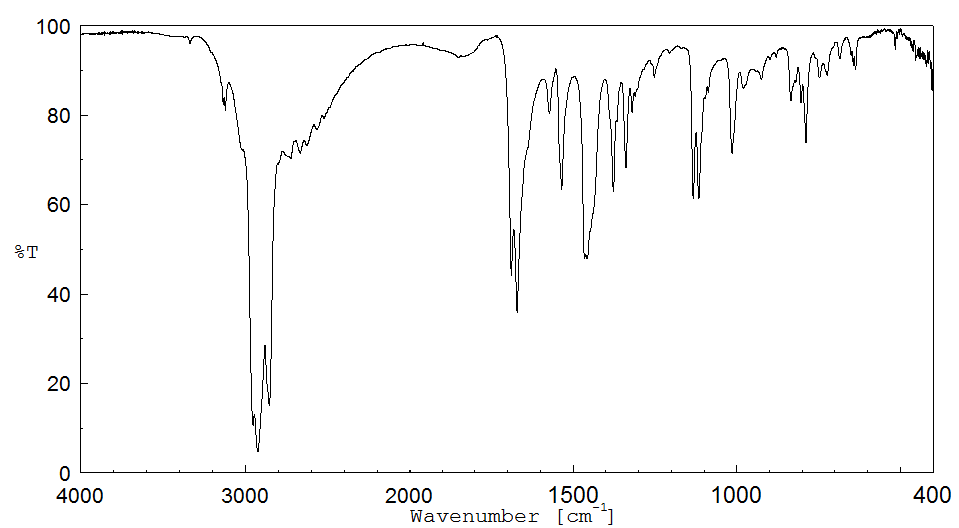
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)