1,3-二硫烷-2-甲酸乙酯 | 20461-99-8
中文名称
1,3-二硫烷-2-甲酸乙酯
中文别名
1,3-二硫戊环-2-羧酸乙酯;1,3-二硫戊环-2-甲酸乙酯
英文名称
ethyl 1,3-dithiolane-2-carboxylate
英文别名
[1,3]dithiolane-2-carboxylic acid ethyl ester;ethyl 2,2-(1,3-dithiolane-2-yl)acetate;Ethyl-1,3-dithiolan-2-carboxylat;2-ethoxycarbonyl-1,3-dithiolane;[1,3]Dithiolan-2-carbonsaeure-aethylester
CAS
20461-99-8
化学式
C6H10O2S2
mdl
MFCD00005411
分子量
178.276
InChiKey
OMCSHTHLIQOHDD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:85 °C/0.1 mmHg (lit.)
-
密度:1.249 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
在常温常压下稳定,应避免接触强氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:76.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S23,S24/25
-
WGK Germany:3
-
海关编码:2934999090
-
危险品运输编号:3334
-
储存条件:将物品存放在紧密的容器中,并储存在阴凉、干燥的地方。
SDS
1.1 产品标识符
: Ethyl 1,3-dithiolane-2-carboxylate
化学品俗名或商品名
1.2 鉴别的其他方法
Glyoxylic acid ethyl ester ethylene mercaptal
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Glyoxylic acid ethyl ester ethylene mercaptal
别名
: C6H10O2S2
分子式
: 178.27 g/mol
分子量
成分 浓度
Ethyl 1,3-dithiolane-2-carboxylate
-
化学文摘编号(CAS No.) 20461-99-8
EC-编号 243-837-9
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
对光线敏感 充气保存
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 淡黄
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
85 °C 在 0.1 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
1.249 g/mL 在 25 °C
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: Ethyl 1,3-dithiolane-2-carboxylate
化学品俗名或商品名
1.2 鉴别的其他方法
Glyoxylic acid ethyl ester ethylene mercaptal
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Glyoxylic acid ethyl ester ethylene mercaptal
别名
: C6H10O2S2
分子式
: 178.27 g/mol
分子量
成分 浓度
Ethyl 1,3-dithiolane-2-carboxylate
-
化学文摘编号(CAS No.) 20461-99-8
EC-编号 243-837-9
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
对光线敏感 充气保存
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 淡黄
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
85 °C 在 0.1 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
1.249 g/mL 在 25 °C
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 1,3-dithiolane-2-carboxylate 180676-69-1 C5H8O2S2 164.249 1,3-二噻戊环-2-羧酸 1,3-dithiolane-2-carboxylic acid 5616-65-9 C4H6O2S2 150.222
反应信息
-
作为反应物:描述:1,3-二硫烷-2-甲酸乙酯 在 1,3,2-oxazaborolidin-5-one 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 20.5h, 生成 ethyl (3R)-2,2-(ethylenedithio)-3-hydroxy-4-methylpentanoate参考文献:名称:基于手性 1,3,2-Oxazaborolidin-5-one 促进的不对称醛醇反应和非对映选择性自由基还原的本质上对映纯素和反丙酸酯醛醇加合物的不同合成摘要:本质上,对映纯的合成和反丙醇醛加合物是使用一种新的策略不同地合成的,该策略利用高度对映选择性的 1,3,2-oxazaborolidin-5-one-促进的醛醇反应与衍生自乙基 2-溴的乙烯酮甲硅烷基缩醛丙酸盐和高度非对映选择性的自由基脱溴反应。这些程序提供的产率增加到可用于实际合成的水平。DOI:10.1246/bcsj.74.1485
-
作为产物:描述:参考文献:名称:Lissel, Manfred, Liebigs Annalen der Chemie, 1982, # 9, p. 1589 - 1601摘要:DOI:
文献信息
-
Synthesis of Unsymmetrical Tetrathiafulvalene Derivatives via Me<sub>3</sub>Al-Promoted Reactions of Organotin Compounds with Esters作者:Jun-ichi Yamada、Shyûji Satoki、Sachinori Mishima、Nobutaka Akashi、Kouhei Takahashi、Nobuyuki Masuda、Yasushi Nishimoto、Satoshi Takasaki、Hiroyuki AnzaiDOI:10.1021/jo952255e日期:1996.1.1In addition, the synthesis of diselenadithiafulvalene derivatives (25-28) could be accomplished by Me(3)Al-mediated reaction of tin thiolate (2a) or selenolates (3d and 5) with esters (22a, 22d, and 24). Furthermore, the application of the Me(3)Al-promoted reaction of tin thiolate (34) with esters (11a-b, 22a-d, and 35a-b) for the synthesis of unsymmetrical TTFs-fused donors enabled us to obtain various
-
General Strategy for the Syntheses of Corynanthe, Tacaman, and Oxindole Alkaloids作者:Alexander Deiters、Martin Pettersson、Stephen F. MartinDOI:10.1021/jo061032t日期:2006.8.1We report herein the total synthesis of the corynanthe alkaloid dihydrocorynantheol and the formal syntheses of the indole alkaloids tacamonine, rhynchophylline, and hirsutine. The strategies for assembling the corynanthe and tacaman skeletal frameworks comprised of both the classical ABD → ABCD and ABC → ABCD approaches wherein the variously substituted piperidinone D-rings were formed via ring-closing我们在此报告了七氢萘生物碱二氢七氢萘酚的总合成以及吲哚生物碱他卡莫宁,乙酰胆碱和hirsutine的形式合成。由经典的ABD→ABCD和ABC→ABCD方法组成的corynanthe和tacaman骨架骨架的组装策略,其中各种取代的哌啶酮D环是通过闭环复分解(RCM)形成,然后加成1,4-在C(15)处引入必要的侧链。由于向α,β-不饱和内酰胺中添加1,4-代表了一个不发达的领域,我们对两种不饱和内酰胺进行了一系列研究,采用有机铜酸盐和金属烯醇盐作为亲核试剂。这些研究表明,源自格氏试剂的有机铜和化学计量的CuCN或催化量的CuBr·DMS络合物都是极好的亲核试剂。TMSC1是优化这些反应的关键添加剂。在这些1,4-加成中,衍生自1,3-二硫杂环戊基-2-羧酸乙酯的阴离子也是极好的亲核试剂,尽管将此类1,4-加成的立体化学反应衍生自咔啉衍生的不饱和内酰胺。吲哚氮原子。这些生物碱的ABD→AB
-
A <i>trans</i>-AB-Bacteriochlorin Building Block作者:Olga Mass、Jonathan S. LindseyDOI:10.1021/jo201967k日期:2011.11.18-one). Here, four new results are reported concerning the synthesis of substituted bacteriochlorins. First, a new, scalable route to 1,1-dimethoxy-4-methylpent-3-en-2-one removes a significant previous impediment to the overall route. Second, the new route was employed to gain access to new α,β-unsaturated ketones and corresponding dihydrodipyrrins bearing alternative substituents in place of the dimethoxy合成细菌氯霉素在光化学领域的基础研究中很受关注,因为它们在近红外光谱区域具有很强的吸收能力,并且与天然细菌叶绿素具有相似的相似性。从头开始生成5-甲氧基细菌绿素的方法需要使二氢联吡啶-乙缩醛自缩合,而缩合又由2-(2-硝基乙基)吡咯和一个α,β-不饱和酮-乙缩醛(例如1,1 -二甲氧基-4-甲基戊-3-烯-2-酮)。在此,报道了关于取代的细菌绿素的合成的四个新结果。首先,一条新的,可扩展的生产1,1-二甲氧基-4-甲基戊-3-烯-2-酮的路线消除了以前对整个路线的重大障碍。其次,采用了新的途径来获得新的α,β-不饱和酮和带有替代取代基的取代二甲氧基单元的二氢二吡咯啉。第三,带有1,3-二氧戊环-2-基部分的二氢二吡喃提供了在5-位含有2-羟基乙氧基取代基的细菌二氢卟啉(30%收率)。第四,随后的溴化反应在15位区域选择性进行,反式-(5,15)-AB-细菌氯霉素构件。线性的5,15取代模式
-
BDH-TTP as a Structural Isomer of BEDT-TTF, and Its Two-Dimensional Hexafluorophosphate Salt作者:Jun-ichi Yamada、Maki Watanabe、Hiroyuki Anzai、Hiroyuki Nishikawa、Isao Ikemoto、Koichi KikuchiDOI:10.1002/(sici)1521-3773(19990315)38:6<810::aid-anie810>3.0.co;2-i日期:1999.3.15Metallic behavior down to low temperature is shown by charge transfer salts of BDH-TTP (1), a structural isomer of bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF). In the case of (BDH-TTP)2 PF6 this behavior is attributed to the structure, which is made up of κ-type sheets of BDH-TTP donor molecules and sheets of PF6- anions.
-
Conjugate Additions to Phenylglycinol-Derived Unsaturated δ-Lactams. Enantioselective Synthesis of Uleine Alkaloids作者:Mercedes Amat、Maria Pérez、Núria Llor、Carmen Escolano、F. Javier Luque、Elies Molins、Joan BoschDOI:10.1021/jo0487101日期:2004.12.1sulfur-stabilized anions) to the phenylglycinol-derived unsaturated lactams trans-2, cis-2, and its 8-ethyl-substituted analogue 10 is studied. The factors governing the exo or endo facial stereoselectivity are discussed. This methodology provides short synthetic routes to either cis- or trans-3,4-disubstituted enantiopure piperidines as well as efficient routes for the enantioselective construction of the tetracyclic
表征谱图
-
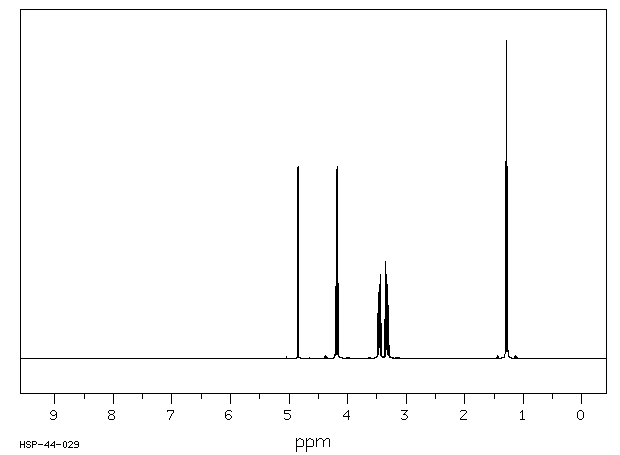
氢谱1HNMR
-
质谱MS
-
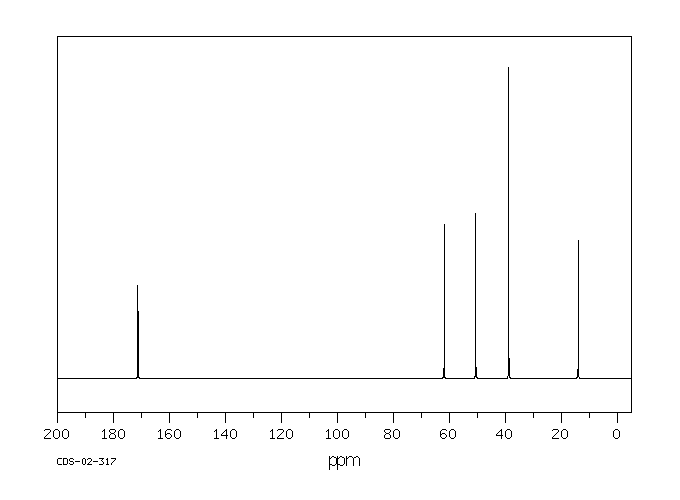
碳谱13CNMR
-
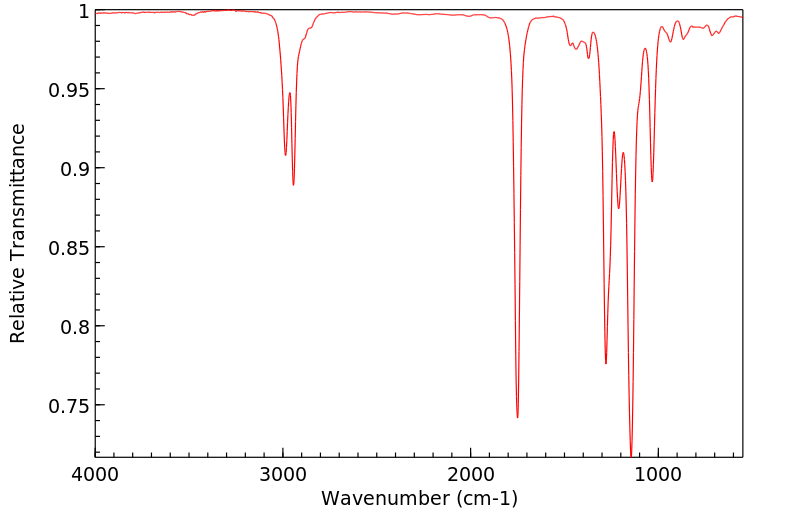
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
螺[二环[2.2.1]庚烷-2,2'-[1,3]二噁戊环]-5-乙醇,(1S,4R,5R)-
芦笋酸
硫辛酸钠
硫辛酸氨基丁三醇盐
硫辛酸杂质D
硫辛酸杂质9
硫辛酸乙酯
硫辛酸-二聚乙二醇-马来酰亚胺
硫辛酰氨基-PEG12-羧酸
甲基沙蚕毒素
沙蚕毒素
氨基乙醛乙烷二硫代缩醛
左旋硫辛酸
呋喃-2-甲醛乙烷-1,2-二基二硫代缩醛
二乙基硫辛酰胺
三硫代碳酸乙烯酯
rac-α-硫辛酸-d5
R-(alpha)-硫辛酸氨基丁三醇盐
R-(+)-硫辛酸
N-(1,3-二噻戊环-2-亚基氨基)乙酰胺
N-(1,3-二噻戊环-2-亚基氨基)-2-氧代丙酰胺
L-赖氨酸单-1,2-二噻戊环-3-戊酸盐
DL-α-硫辛酸-NHS
5-[(3R)-二噻戊环-3-基]戊酸;2-羟基丙酸
4-甲基二噻戊环-3-酮
4-甲基-1,3-二硫醇-2-酮
4-甲基-1,3-二噻戊环-2-亚胺盐酸盐
4-甲基-1,2-噻吩-4-羧酸
4-甲基-1,2-二噻吩-4-羧胺
4-噻唑烷酮,3-(二甲氨基)-2-亚硫酰基-,(Z)-
4-乙基-1,3-二噻戊环-2-硫酮
4-[[5-(1,2-二噻戊环-3-基)-1-氧代戊基]氨基]丁酸
4-[(苯基硫基)甲基]苯甲酸
4,5-二甲基-2-[2-(甲硫基)乙基]-1,3-二噻戊环
3-环丁烯砜-D6
2-甲基-1,3-二硫戊环
2-异丙基-4-甲基-1,3-二噻戊环
2-己基-1,3-二噻戊环
2-亚甲基-1,3-二硫杂环戊烷
2-(氯甲基)-1,3-二噻戊环
2-(三氯甲基)-1,3-二噻戊环
2-(2-噻吩基)-1,3-二噻戊环
2-(2,4-环戊二烯-1-亚基)-1,3-二硫戊环
2-(1,3-二噻戊环-2-基)-1,3-二噻戊环
2-(1,2-二硫烷-3-基)乙酸
2,4-二氯-6,7-二硫杂双环[3.2.1]辛烷
2,3-二硫杂螺[4.4]壬烷
2,3,7,8-四硫杂螺[4.4]壬烷
2,2'-[1,2-乙烷二基二(硫代)]二[2-(三氟甲基)-1,3-二噻戊环]
1,‐2-二硫戊基-4-醇