β-氯醛糖 | 16376-36-6
中文名称
β-氯醛糖
中文别名
1,2-O-[(1S)-2,2,2-三氯亚乙基]-ALPHA-D-呋喃葡萄糖;β-葡萄糖缩氯醛;Β-三氯乙醛化葡萄糖
英文名称
β-chloralose
英文别名
β-glucochloralose;beta-Chloralose;(1R)-1-[(2S,3aR,5R,6S,6aR)-6-hydroxy-2-(trichloromethyl)-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3]dioxol-5-yl]ethane-1,2-diol
CAS
16376-36-6
化学式
C8H11Cl3O6
mdl
——
分子量
309.531
InChiKey
OJYGBLRPYBAHRT-GVUNPQSCSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:234-236 °C (dec.)(lit.)
-
沸点:504.4±45.0 °C(Predicted)
-
密度:1.773
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:88.4
-
氢给体数:3
-
氢受体数:6
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S36
-
危险类别码:R20/21/22
-
WGK Germany:3
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:UN 2811
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— α-chloralose 39598-39-5 C8H11Cl3O6 309.531 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5,6-O-isopropylidene-1,2-O-trichloroethylidene-α-D-glucofuranose 88390-56-1 C11H15Cl3O6 349.596 —— (S)-1,2-O-(2,2,2-trichloroethylidene)-α-D-xylo-1,4-furanodialdose 124595-32-0 C7H7Cl3O5 277.489 —— 5-(E)-eno-3-O-methyl-1,2-O-(S)-trichloroethylidene-5,6,8-trideoxy-α-D-xylo-1,4-furano-7-ulose 872321-65-8 C11H13Cl3O5 331.58 —— 6-O-tosyl-1,2-O-(S)-trichloroethylidene-α-D-glucofuranose 1239975-47-3 C15H17Cl3O8S 463.72
反应信息
-
作为反应物:描述:β-氯醛糖 在 吡啶 、 sodium periodate 作用下, 以 甲醇 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 2.0h, 生成 3-O-acetyl-(S)-1,2-O-trichloroethylidene-5,6,8-trideoxy-α-D-xylo-oct-5(E)-eno-1,4-furano-7-ulose参考文献:名称:1,2-O-三氯亚乙基缩醛基保护的3,5-二烯-1,4-呋喃糖衍生物。摘要:3,5-(E)-dieno-3,5,6,8-四脱氧-(S)-1,2-O-三氯亚乙基-α-D-甘油-octo-1,4-呋喃酮-7的制备从1,2-O-(S)-三氯亚乙基-α-D-葡萄糖基呋喃糖(β-氯醛糖)或1,2-O-(S)-三氯亚乙基-α-D-半乳糖呋喃糖(半乳糖氯醛)开始3,5-(E)-dieno-3,5,6-trideoxy-(S)-1,2-O-trichloroethylidene-alpha-D-glycerome-hepta-1,4-furano-uronate的制备描述了β-氯藻糖。内环双键的形成是通过使用DMF-碳酸氢钠消除3-乙酰氧基来实现的。当起始化合物为1,2-O-(R)-三氯亚乙基-α-D-葡萄糖呋喃糖(α-氯醛糖)(其中三氯甲基占据内位)时,这种消除不成功。DOI:10.1016/s0008-6215(03)00337-9
-
作为产物:参考文献:名称:Glucofuranose derivatives as a library for designing and investigating low molecular mass organogelators摘要:We designed and synthesized a class of saccharide-based gelators having three free OH groups in the glucofuranose fragment. The gelating abilities of fourteen compounds were examined to systematically study the influence of the hydrophobic fragment connected to the C2' carbon. Also the correlation between the saccharide crystal structure and its gelating properties was examined, showing limited usefulness in this particular case. SEM observations were carried out in order to investigate the hierarchical structure of xerogels and changes depending on different gel concentration. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2005.07.093
文献信息
-
(METH)ACRYLATE MANUFACTURING METHOD申请人:TOAGOSEI CO., LTD公开号:US20180118658A1公开(公告)日:2018-05-03The present invention provides a (meth)acrylate manufacturing method characterized in that when manufacturing a (meth) acrylate by an ester exchange reaction between an alcohol and a monofunctional (meth)acrylate using catalyst A and catalyst B together, contact treatment of the ester exchange reaction product with adsorbent C is performed. Catalyst A: One or more kinds of compounds selected from a group consisting of cyclic tertiary amines with an azabicyclo structure and salts or complexes thereof, amidine and salts or complexes thereof, compounds with a pyridine ring and salts or complexes thereof, phosphines and salts or complexes thereof, and compounds with a tertiary diamine structure and salts or complexes thereof. Catalyst B: One or more kinds of compounds selected from a group consisting of compounds comprising zinc. Adsorbent C: One or more kinds of compounds selected from a group consisting of oxides and hydroxides comprising at least one of magnesium, aluminum and silicon.
-
MULTIFUNCTIONAL (METH)ACRYLATE MANUFACTURING METHOD申请人:TOAGOSEI CO., LTD.公开号:US20170204044A1公开(公告)日:2017-07-20[Problem] The purpose of the present invention is to obtain a multifunctional (meth)acrylate with good yield by an ester exchange reaction of a polyhydric alcohol such as pentaerythritol or dipentaerythritol with a monofunctional (meth)acrylate. [Solution] A multifunctional (meth)acrylate manufacturing method characterized in that when manufacturing a multifunctional (meth)acrylate by an ester exchange reaction of a polyhydric alcohol with a monofunctional (meth)acrylate, catalyst (A) and catalyst (B) are used together. Catalyst (A): One or more kinds of compounds selected from a group consisting of cyclic tertiary amines with an azabicyclo structure or salts or complexes thereof, amidines or salts or complexes thereof, and compounds with a pyridine ring or salts or complexes thereof. Catalyst (B): One or more kinds of compounds selected from a group consisting of zinc-containing compounds.
-
METHOD FOR PRODUCING (METH)ACRYLATE申请人:TOAGOSEI CO., LTD.公开号:US20180105483A1公开(公告)日:2018-04-19A method for producing a (meth) acrylate comprises transesterification reaction of an alcohol and a monofunctional (meth) acrylate with catalysts in combination being cyclic tertiary amines having an azabicyclo structure and compounds containing zinc, separating a solid that contains the catalysts from a reaction product containing a (meth) acrylate, and producing a (meth) acrylate by transesterification reaction of an alcohol and a monofunctional (meth) acrylate, while using the recovered solid catalyst.
-
The Knoevenagel-Doebner Reaction on 1,2-O-(2,2,2-Trichloroethylidene) Derivatives of D-Gluco- and D-Manno- furanose作者:Gökhan Kök、Tamer Karayıldırım、Kadir Ay、Emriye AyDOI:10.3390/molecules15117724日期:——furanuronic acid derivatives of α-gluco- (3), β-gluco- (6) and β-manno-chloraloses (9) via a convenient one pot procedure using the Knoevenagel-Doebner reaction approach are described. The dialdofuranose derivatives were reacted with malonic acid under Knoevenagel-Doebner reaction conditions and (E)-α,β-unsaturated furanuronic acid derivatives were obtained.
-
Herbicidal composition containing dioxolane, dioxane, or dioxepane申请人:Magyar Tudomanvos Akademia Kozponti Kemiai Kutato Intezete Nitrokemia公开号:US05116402A1公开(公告)日:1992-05-26The invention relates to a herbicidal composition which contains, besides binding, wetting, dispersing, emulsifying agents, solvents and/or surface-active substances, herbicides as active agent of thiocarbamate, carbamate, acid amide or urea type alone or in a combination, furthermore as antidote a compound of the general formula I ##STR1## wherein R.sup.1 and R.sup.2, independently of each other, are hydrogen, C.sub.1-6 alkyl, C.sub.2-6 alkenyl, C.sub.1-4 cyanoalkyl, C.sub.1-6 haloalkyl, phenyl-C.sub.1-4 -haloalkyl, phenyl, halogenphenyl, C.sub.1-4 -alkyl-phenyl, C.sub.1-4 -alkoxyphenyl, furfuryl; R.sup.3 and R.sup.4, independently of each other, are hydrogen, C.sub.1-18 -alkyl, C.sub.2-4 -haloalkyl, C.sub.2-4 -cyanoalkyl, C.sub.1-4 -alkoxy-C.sub.2-4 -alkyl, C.sub.5-6 -cycloalkyl, phenyl-C.sub.1-4 -alkyl, C.sub.3-4 -alkenyl, phenyl-C.sub.3-4 -alkenyl, di-C.sub.1-4 -alkylamino-C.sub.2-4 -alkyl, hydroxy-C.sub.2-6 -alkyl, furfuryl, tetrahydrofurfuryl, C.sub.1-4 -alkoxy-C.sub.2-4 -alkoxy-C.sub.2-4 -alkyl; R.sup.3 and R.sup.4, together, are C.sub.2-4 -alkylene, C.sub.4 -alkenylene, glucofuranosylene, acetoxy-C.sub.3 -alkylene, C.sub.1-4 -alkoxy-C.sub.3 -alkylene, hydroxy-C.sub.3 -alkylene, halogen-C.sub.3 -alkylene; wherein the quantity of the antidote lies between 0.01 and 15 parts by weight referred to 1 part by weight of herbicidal agent, furthermore the composition contains altogether 0.1 to 95 percent by weight of the herbicidal agents and the antidote.本发明涉及一种除草剂组合物,除含有粘合剂、润湿剂、分散剂、乳化剂、溶剂和/或表面活性物质外,还含有硫代氨基甲酸酯、氨基甲酸酯、酸酰胺或脲类除草剂作为活性剂,单独或组合使用,此外还含有一种通式I的抗毒剂: ##STR1## 其中,R1和R2独立地为氢、C1-6烷基、C2-6烯基、C1-4氰基烷基、C1-6卤代烷基、苯基-C1-4-卤代烷基、苯基、卤代苯基、C1-4-烷基-苯基、C1-4-烷氧基苯基、呋喃基;R3和R4独立地为氢、C1-18烷基、C2-4卤代烷基、C2-4氰基烷基、C1-4-烷氧-C2-4-烷基、C5-6环烷基、苯基-C1-4-烷基、C3-4-烯基、苯基-C3-4-烯基、二-C1-4-烷基氨基-C2-4-烷基、羟基-C2-6-烷基、呋喃基、四氢呋喃基、C1-4-烷氧-C2-4-烷氧-C2-4-烷基;R3和R4在一起为C2-4-烷基、C4-烯基、葡萄糖呋喃糖基、乙酰氧-C3-烷基、C1-4-烷氧-C3-烷基、羟基-C3-烷基、卤代-C3-烷基;其中抗毒剂的数量在除草剂的1份重量中为0.01至15份重量,此外,该组合物总含量为0.1至95重量百分比的除草剂和抗毒剂。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
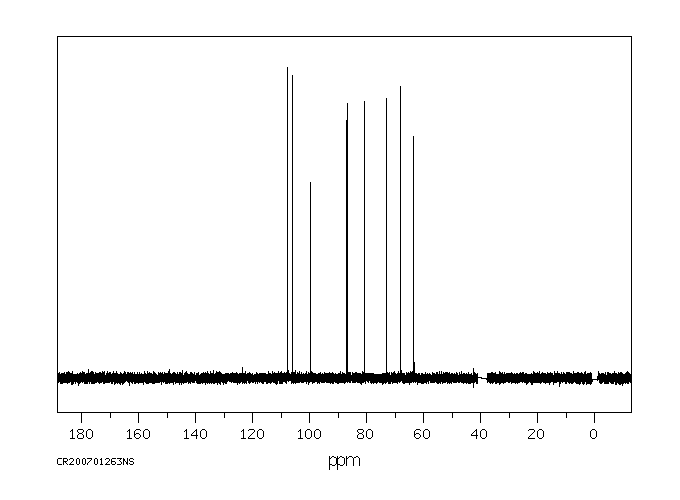
碳谱13CNMR
-
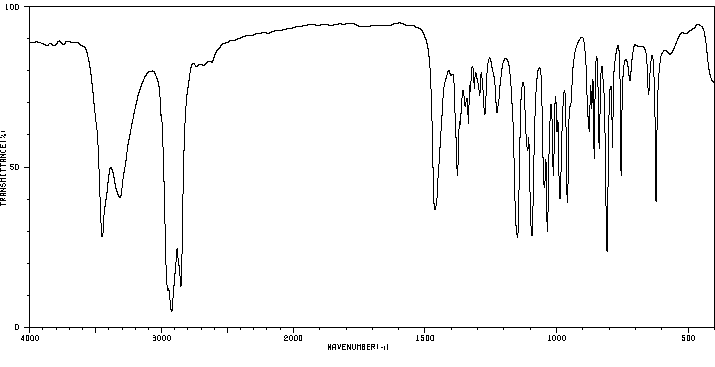
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷