4,8-二甲基壬-3,7-二烯-2-醇 | 67845-50-5
中文名称
4,8-二甲基壬-3,7-二烯-2-醇
中文别名
——
英文名称
4,8-dimethylnona-3,7-dien-2-ol
英文别名
4,8-dimethyl-3,7-nonadien-2-ol;8-hydroxy-2,6-dimethyl-nona-2,6-diene;3,7-Nonadien-2-ol, 4,8-dimethyl-
CAS
67845-50-5
化学式
C11H20O
mdl
——
分子量
168.279
InChiKey
NYPOJSCNHYUZRG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:105-106 °C(Press: 18 Torr)
-
密度:0.863±0.06 g/cm3(Predicted)
-
LogP:3.63
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:12
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.64
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,7-二甲基-2,6-辛二烯-1-醇 geraniol 624-15-7 C10H18O 154.252 柠檬醛 (E/Z)-3,7-dimethyl-2,6-octadienal 5392-40-5 C10H16O 152.236 (E)-3,7-二甲基-2,6-辛二烯醛 3,7-dimethyl-2,6-octadienal 141-27-5 C10H16O 152.236
反应信息
-
作为反应物:描述:4,8-二甲基壬-3,7-二烯-2-醇 在 叔丁基过氧化氢 、 4-二甲氨基吡啶 、 copper acetylacetonate 、 titanium tetra-n-propoxide 、 二甲基硫 、 臭氧 、 间氯过氧苯甲酸 、 N,N'-二环己基碳二亚胺 作用下, 以 二氯甲烷 为溶剂, 反应 2.5h, 生成 (R)-(-)-3,7-dimethyl-3-(4-methoxyphenyl)octa-1,6-diene参考文献:名称:Synthesis of (S)- and (R)-Sporochnol by Using the Allylic Substitution of the Secondary Allylic Picolinate摘要:The allylic substitution of secondary allylic picolinates and copper reagents for the construction of a quaternary carbon was applied to synthesis of sporochnol. The enantiomerically enriched allylic picolinate (R)-5 was synthesized through the asymmetric hydrogen transfer of acetylene ketone 11 and the Pd-catalyzed methylation of the iodoallylic alcohol 16a. The key allylic substitution of the allylic picolinate (R)-5 with 4-MeOC6H4MgBr/Cu(acac)(2) (2:1) proceeded with 95% chirality transfer with 98% regioselectivity to afford anti S(N)2' product 6 in 89% yield, which was converted to the methyl ether of unnatural (R)-sporochnol. Similarly, the methyl ether of (S)-sporochnol (the natural form) was synthesized.DOI:10.3987/com-15-s(t)11
-
作为产物:描述:柑橘酮 在 dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer 、 C21H28N2O2S 、 sodium isopropylate 、 lithium chloride 作用下, 以 异丙醇 为溶剂, 反应 2.0h, 生成 4,8-二甲基壬-3,7-二烯-2-醇参考文献:名称:Fine-tuning catalytic activity and selectivity—[Rh(amino acid thioamide)] complexes for efficient ketone reduction摘要:Amino acid-derived thioamides are prepared and evaluated as ligands in the rhodium-catalyzed asymmetric transfer hydrogenation of ketones in 2-propanol. It is found that increasing the steric bulk at the C-terminus of the ligand had a positive impact on both activity and selectivity in the reduction reaction. In order to find the optimum catalyst, a study is performed on a series of thioamide ligands having substituents of varying size. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2009.08.116
文献信息
-
The Cinchona Primary Amine-Catalyzed Asymmetric Epoxidation and Hydroperoxidation of α,β-Unsaturated Carbonyl Compounds with Hydrogen Peroxide作者:Olga Lifchits、Manuel Mahlau、Corinna M. Reisinger、Anna Lee、Christophe Farès、Iakov Polyak、Gopinadhanpillai Gopakumar、Walter Thiel、Benjamin ListDOI:10.1021/ja402058v日期:2013.5.1Using cinchona alkaloid-derived primary amines as catalysts and aqueous hydrogen peroxide as the oxidant, we have developed highly enantioselective Weitz-Scheffer-type epoxidation and hydroperoxidation reactions of α,β-unsaturated carbonyl compounds (up to 99.5:0.5 er). In this article, we present our full studies on this family of reactions, employing acyclic enones, 5-15-membered cyclic enones, and
-
Copper-Catalyzed One-Pot Borylative Aldolisation β-Fluoride Elimination for the Formal Addition of Acrylates to Carbonyl Moieties作者:Corentin Rasson、Adrien Stouse、Arnaud Boreux、Virginie Cirriez、Olivier RiantDOI:10.1002/chem.201802023日期:2018.7.2borylation/aldolisation of methyl 2‐fluoroacrylate with carbonyl compounds followed by an elimination to give Morita–Baylis–Hillman (MBH) analogues. The optimal conditions described were shown to be compatible with a wide range of aldehydes and ketones. Unprecedented MBH adducts derived from ketones were efficiently synthesized.
-
Action des dialkylcuprates de lithium sur les aldéhydes α,β -éthylénioues作者:C. Chuit、J.P. Foulon、J.F. NormantDOI:10.1016/0040-4020(80)80126-8日期:1980.1Nearly exclusive 1-4 addition produts obtained by action of lithium dialkylcuprates with α,β ethylenic aldehydes. Non polar solvents and low temperatures favour this reaction .Only α,β-ehylenic aldehydes having a trisubstituted double bond give a relatively important proportation of 1–2 addition product.
-
Cyclopropanation with Dibromomethane under Grignard and Barbier Conditions作者:Fridtjof Schröder、Gerhard Brunner、Laura Eberhard、Jürg OetikerDOI:10.1055/s-0029-1216999日期:2009.11from α-substituted (homo)allyl alcohols. In tandem reactions, cyclopropyl carbinols are obtained from allyloxylithium or -magnesium intermediates, generated in situ by alkylation of conjugated aldehydes, ketones, and esters as well as from allyl esters and carbonates or vinyloxiranes. [¹] cyclopropanation - Barbier conditions - Grignard addition - alkyllithium addition - dibromomethane
-
Pd(II)−Hydrotalcite-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones Using Atmospheric Pressure of Air作者:Nobuyuki Kakiuchi、Yasunari Maeda、Takahiro Nishimura、Sakae UemuraDOI:10.1021/jo010338r日期:2001.10.1acetate-pyridine complex supported by hydrotalcite) catalyzes the aerobic oxidation in toluene of a variety of primary and secondary alcohols into the corresponding aldehydes and ketones in high yields using atmospheric pressure of air as a sole oxidant under mild conditions. This catalyst is also effective for the oxidation of allylic alcohols, especially such as geraniol and nerol, without any isomerization of an
表征谱图
-
氢谱1HNMR
-
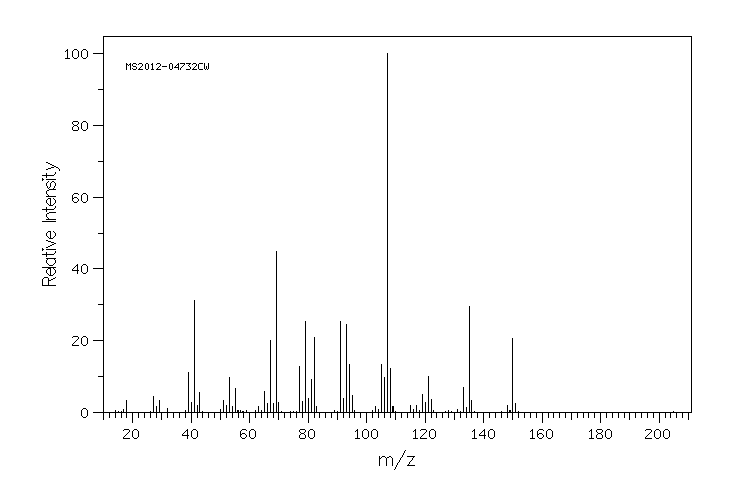
质谱MS
-
碳谱13CNMR
-
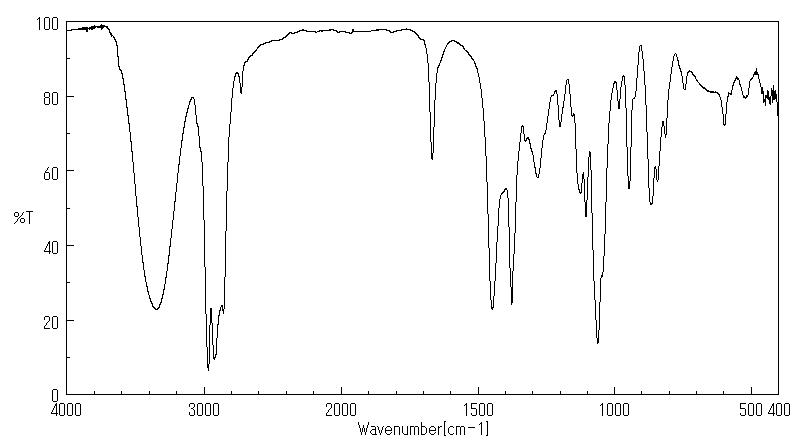
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸