1,2-环氧-7-辛烯 | 19600-63-6
中文名称
1,2-环氧-7-辛烯
中文别名
1,2-环氧基-7-辛烯
英文名称
1,2-epoxy-7-octene
英文别名
2-(hex-5-en-1-yl)oxirane;1,2-epoxyoct-7-ene;2-hex-5-enyloxirane
CAS
19600-63-6
化学式
C8H14O
mdl
MFCD00005156
分子量
126.199
InChiKey
UKTHULMXFLCNAV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:64-65°C 18mm
-
密度:0.85
-
闪点:38 °C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
在常温常压下稳定,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:9
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3
-
危险品标志:Xi
-
安全说明:S16,S18,S26,S36/37/39,S37/39
-
危险类别码:R41
-
海关编码:2910900090
-
危险品运输编号:1993
-
RTECS号:RR0502000
-
包装等级:III
-
危险类别:3
-
储存条件:将温度和存储条件置于同一行,可以更清晰地表达信息: 0-6°C,密闭保存,在阴凉干燥处。
SDS
| Name: | 1 2-Epoxy-7-octene 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 19600-63-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 19600-63-6 | 1,2-Epoxy-7-octene | 97% | 243-178-7 |
Risk Phrases: 10 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Refrigerator/flammables.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 19600-63-6: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 38 deg C ( 100.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8540g/cm3
Molecular Formula:
Molecular Weight: 126.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 19600-63-6: RR0502000 LD50/LC50:
Not available.
Carcinogenicity:
1,2-Epoxy-7-octene - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 10 Flammable.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 19600-63-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 19600-63-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 19600-63-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-1,2-epoxy-7-octene 105205-70-7 C8H14O 126.199 —— (2S)-(2-hex-5-enyl)oxirane 52485-73-1 C8H14O 126.199 1,2-环氧辛烷 1,2-Epoxyoctane 2984-50-1 C8H16O 128.214 1,2,7,8-二环氧辛烷 1,2,7,8-diepoxyoctane 2426-07-5 C8H14O2 142.198 —— 7-octen-2-ol 39546-75-3 C8H16O 128.214 —— 1,8-nonadien-3-ol 159010-02-3 C9H16O 140.225 7,8-二羟基-1-辛烯 7-octene-1,2-diol 85866-02-0 C8H16O2 144.214 —— (S)-oct-7-ene-1,2-diol —— C8H16O2 144.214 —— (2R)-oct-7-en-1,2-diol 119066-31-8 C8H16O2 144.214 8-壬烯-1,2-二醇 8-nonen-1,2-diol 139035-30-6 C9H18O2 158.241 —— 1-fluorooct-7-en-2-ol 127296-12-2 C8H15FO 146.205 —— 4-hydroxy-9-decenenitrile 185313-68-2 C10H17NO 167.251 - 1
- 2
反应信息
-
作为反应物:描述:1,2-环氧-7-辛烯 在 二甲基亚砜 、 bismuth mandelate 作用下, 反应 24.0h, 以50%的产率得到6-庚烯酸参考文献:名称:Bi(III)-扁桃酸盐/ DMSO:一种新的氧化体系,用于催化环氧化物的CC裂解摘要:发现扁桃酸铋(III)是在无水DMSO介质中氧化环氧化物的CC键裂解和将其转化为羧酸的有效催化剂。DOI:10.1016/s0040-4039(00)77635-0
-
作为产物:参考文献:名称:钴(II)催化烯烃与脂肪醛和分子氧的反应:范围和机理摘要:可以使用衍生自芳族醛和胺或α-氨基酯的席夫碱制备各种钴(II)配合物。这些络合物是脂肪族醛与各种烯烃之间反应的通用催化剂。反应的结果受烯烃的电子性质控制,因为缺电子的烯烃经历醛的氧化加成反应,然后引入双氧生成2-羟基(酰氧基)-4-氧酸酯或腈,而未活化或富电子的烯烃则提供了相应的环氧化物。这些反应是通过自由基途径进行的,并提出了一种常见的酰基钴中间体,用于形成4和环氧化物7。DOI:10.1016/s0040-4020(01)87194-5
文献信息
-
Free-Radical Bromotrifluoromethylation of Olefin via Single-Electron Oxidation of NaSO<sub>2</sub>CF<sub>3</sub> by NaBrO<sub>3</sub>作者:Zhong-Quan Liu、Dong LiuDOI:10.1021/acs.joc.6b02812日期:2017.2.3A free-radical bromotrifluoromethylation of olefin by using NaSO2CF3 and NaBrO3 has been achieved. Sodium bromate acts not only as a single-electron oxidant but also as a bromine source. A radical-clock experiment and electron-spin-resonance detection support a radical process.
-
Environmentally friendly catalysis using supported reagents: catalytic epoxidation using a chemically modified silica gel作者:Andrew J. Butterworth、James H. Clark、Paul H. Walton、Simon J. BarlowDOI:10.1039/cc9960001859日期:——A new heterogeneous catalyst based on a chemically modified mesoporous silica gel possessing immobilised cobalt ions is developed and successfully applied to the epoxidation of alkenes.
-
Halogen substituted metallocene compounds for olefin polymerization申请人:Voskoboynikov Z. Alexander公开号:US20070135594A1公开(公告)日:2007-06-14A metallocene compound is represented by the formula (1): AMX n-1 wherein M is a transition metal atom having a coordination number of n selected from Group 3, 4, 5 or 6 of the Periodic Table of Elements, or a lanthanide metal atom, or actinide metal atom; A is a substituted monocyclic or polycyclic arenyl ligand pi-bonded to M, wherein said arenyl ligand includes at least one halogen substituent directly bonded to any sp 2 carbon atom at a bondable ring position of the ligand; and the or each X is a univalent anionic ligand, or two X are joined and bound to the metal atom to form a metallocycle ring, or two X are joined to form a chelating ligand, a diene ligand, or an alkylidene ligand.
-
Catalyst composition for hydrogenation and method for hydrogenation using the same申请人:Asahi Kasei Chemicals Corporation公开号:US10016749B2公开(公告)日:2018-07-10A catalyst composition for hydrogenation including (A) to (D), in which a mass ratio ((C)/(A)) is 0.1 to 4.0 and a mass ratio ((D)/(A)) is 0.01 to 1.00, (A): a titanocene compound represented by formula (1), (wherein R5 and R6 are any group selected from hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, an aryloxy group, an alkoxy group, a halogen group, and a carbonyl group. R1 and R2 are any group selected from the group consisting of hydrogen and a hydrocarbon group having 1 to 12 carbon atoms, and R1 and R2 are not all hydrogen atoms or all a hydrocarbon group having 1 to 12 carbon atoms), (B): a reductant formed from a compound containing an element selected from the elements Li, Na, K, Mg, Zn, Al, and Ca, (C): an unsaturated compound having a molecular weight of 400 or less, and (D): a polar compound.
-
Photoredox Catalysis Mediated Application of Methyl Fluorosulfonyldifluoroacetate as the CF<sub>2</sub>CO<sub>2</sub>R Radical Source作者:Wei Yu、Xiu-Hua Xu、Feng-Ling QingDOI:10.1021/acs.orglett.6b02580日期:2016.10.7A new application of trifluoromethylating reagent, methyl fluorosulfonyldifluoroacetate (FO2SCF2CO2Me, Chen’s reagent), as the carbomethoxydifluoromethylating reagent under visible light photoredox conditions is reported. The visible-light-induced reaction of FO2SCF2CO2Me with unactivated alkenes, styrenes, or heteroarenes affords a variety of carbomethoxydifluoromethylated products.
表征谱图
-
氢谱1HNMR
-
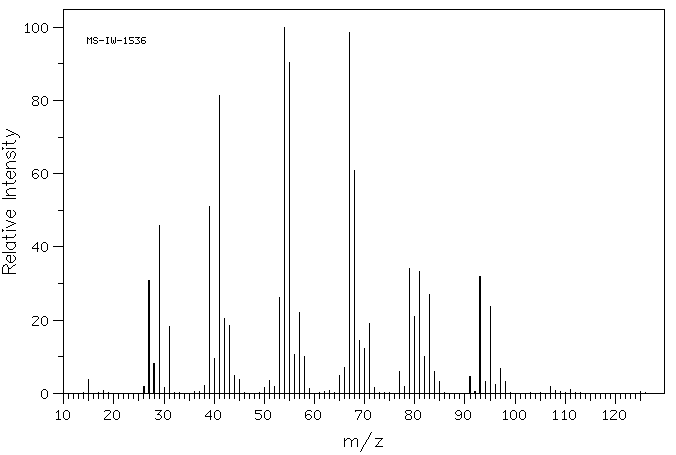
质谱MS
-
碳谱13CNMR
-
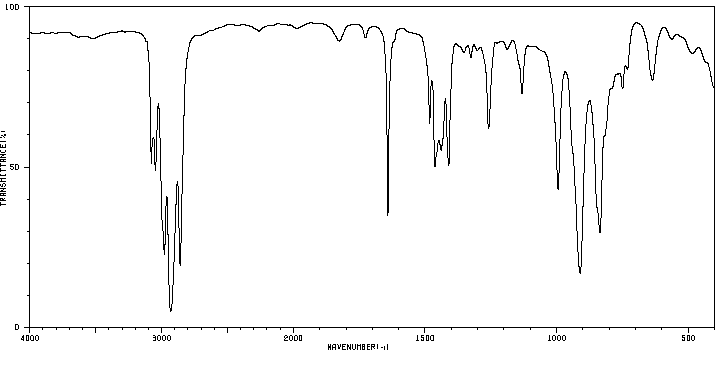
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷