α-(甲氨甲基)苯甲醇 | 6589-55-5
中文名称
α-(甲氨甲基)苯甲醇
中文别名
——
英文名称
2-(methylamino)-1-phenylethanol
英文别名
N-methylphenylethanolamine
CAS
6589-55-5
化学式
C9H13NO
mdl
MFCD00004506
分子量
151.208
InChiKey
ZCTYHONEGJTYQV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-76 °C (lit.)
-
沸点:132-133 °C(Press: 15 Torr)
-
密度:1.02 g/cm3(Temp: 0 °C)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
物理描述:Solid
-
稳定性/保质期:
稳定。不与酸、酸氯化物、酸酐或氧化剂反应。
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:32.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S36
-
危险类别码:R36/37/38,R20/22
-
WGK Germany:3
-
海关编码:29221985
-
储存条件:2-8°C
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
: α-(Methylaminomethyl)benzyl alcohol
Product name
CAS-No. : 6589-55-5
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Inhalation (Category 4)
Acute toxicity, Oral (Category 4)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful by inhalation and if swallowed.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H332 Harmful if inhaled.
Precautionary statement(s) none
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R20/22 Harmful by inhalation and if swallowed.
S-phrase(s)
S36 Wear suitable protective clothing.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : Halostachine
Formula : C9H13NO
Molecular Weight : 151,21 g/mol
Component Concentration
α-[(Methylamino)methyl]benzyl alcohol
CAS-No. 6589-55-5 -
EC-No. 229-525-5
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: crystalline
Colour: white
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 74 - 76 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
acids, Acid chlorides, Acid anhydrides, Oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Intraperitoneal - mouse - 500 mg/kg
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation Harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: DO9625000
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-1-苯乙醇 2-Amino-1-phenylethanol 7568-93-6 C8H11NO 137.181 N-(2-羟基-2-苯基乙基)-N-甲基甲酰胺 α-phenyl-β-(N-methylformamido)ethanol 88953-55-3 C10H13NO2 179.219 —— N-methyl-2-hydroxy-2-phenylethanamide 65645-88-7 C9H11NO2 165.192 N-(2-羟基-2-苯基乙基)-N-甲基亚硝酰胺 (2-hydroxy-2-phenylethyl)methylnitrosamine 36972-73-3 C9H12N2O2 180.206 1-苯基-2-硝基乙醇 2-nitro-1-phenylethan-1-ol 15990-45-1 C8H9NO3 167.164 —— Methyl-(β-trimethylsiloxy-β-phenyl-aethyl)-amin 22081-35-2 C12H21NOSi 223.39 3-甲基-5-苯基-1,3-恶唑烷 3-methyl-5-phenyloxazolidine 88953-56-4 C10H13NO 163.219 2-溴-1-苯乙醇 2-Bromo-1-phenylethanol 2425-28-7 C8H9BrO 201.063 (±)-2-氯-1-苯基乙醇 1-phenyl-2-chloroethanol 1674-30-2 C8H9ClO 156.612 氧化苯乙烯 styrene oxide 96-09-3 C8H8O 120.151 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基(S)-2-羟基-2-苯基乙胺 N-methyl (S)-2-hydroxy-2-phenylethylamine 65058-52-8 C9H13NO 151.208 盐穗草碱 (-)-Halostachine 495-42-1 C9H13NO 151.208 —— (+/-)-2- -1-phenyl-aethanol 5806-63-3 C11H17NO 179.262 N-(2-羟基-2-苯基乙基)-N-甲基甲酰胺 α-phenyl-β-(N-methylformamido)ethanol 88953-55-3 C10H13NO2 179.219 (1S,2S)-2-甲氨基-1-苯基丙-1-醇 pseudoephedrine 90-82-4 C10H15NO 165.235 麻黄素 (1S,2R)-(+)-ephedrine 321-98-2 C10H15NO 165.235 N-(2-羟基-2-苯基乙基)-N-甲基亚硝酰胺 (2-hydroxy-2-phenylethyl)methylnitrosamine 36972-73-3 C9H12N2O2 180.206 —— Methyl-(β-trimethylsiloxy-β-phenyl-aethyl)-amin 22081-35-2 C12H21NOSi 223.39 3-甲基-5-苯基-1,3-恶唑烷 3-methyl-5-phenyloxazolidine 88953-56-4 C10H13NO 163.219 —— 2-[Methyl-[(3-methylphenyl)methyl]amino]-1-phenylethanol 339001-09-1 C17H21NO 255.36 —— 2-[(4-bromo-benzyl)-methyl-amino]-1-phenyl-ethanol 415954-71-1 C16H18BrNO 320.229 - 1
- 2
反应信息
-
作为反应物:描述:α-(甲氨甲基)苯甲醇 在 甲烷磺酸 、 sodium hydride 、 二异丁基氢化铝 、 1-羟基苯并三唑 、 1-(3-二甲基氨基丙基)-3-乙基碳二亚胺 作用下, 以 四氢呋喃 、 二氯甲烷 、 甲苯 为溶剂, 反应 21.0h, 生成 3-methyl-7-(methyloxy)-8-{[(methyloxy)methyl]oxy}-1-phenyl-2,3-dihydro-1H-3-benzazepine参考文献:名称:功能化 2,5-二取代苯并氮杂:3-Methyl-5-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine-2-carbonitrile 和相关衍生物的立体选择性合成摘要:从[4-羟基-3-(甲氧基)苯基]乙酸开始,开发了2-腈和2-氨基甲基取代的5-苯基苯并氮杂衍生物的立体选择性合成。合成中的关键步骤是将氰化物立体选择性加成到 3-methyl-1-phenyl-2,3-dihydro-1H-3-benzazepine (4) 上,得到 15:1 的反式/顺式混合物 2-腈- 5-苯基苯并氮杂非对映异构体5a/5b,产率为83%。通过 1 H NMR NOESY 分析推断主要产物的立体化学。可以通过以立体选择性方式还原成相应的氨甲基衍生物 3a 和 3b 来分离和进一步操作腈非对映异构体。氨甲基苯并氮杂模板 3 有可能用作合成各种在苯并氮杂支架的 2-位修饰的衍生物的手柄,DOI:10.1055/s-2004-825618
-
作为产物:描述:N-methyl-2-hydroxy-2-phenylethanamide 在 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 为溶剂, 反应 8.0h, 以95.6%的产率得到α-(甲氨甲基)苯甲醇参考文献:名称:Moehre, H.; Kamper, Ch., Pharmazie, 1983, vol. 38, # 8, p. 512 - 520摘要:DOI:
-
作为试剂:描述:参考文献:名称:氨基醇引发的四氯甲烷与辛-1-烯的加成反应摘要:研究了由胺、芳香醇和氨基醇(麻黄素的结构类似物)引发的 CCl4 添加到 oct-1-ene 的动力学和机制。该反应的自由基机理是通过 ESR 使用自旋陷阱技术建立的。作为引发剂的芳香氨基醇比具有相似结构的胺和芳香醇更具活性。与胺和芳香醇相比,它们更具选择性,并且在室温下已经与四氯化碳反应,主要形成苯甲醛。在实验数据的基础上,提出了由芳香族氨基醇引发的CCl4向烯烃加成反应的方案。DOI:10.1007/s11172-006-0464-z
文献信息
-
[EN] UREA DERIVATIVES OF AMPHOTERICIN B DERIVED FROM SECONDARY AMINES<br/>[FR] DÉRIVÉS D'URÉE DE L'AMPHOTÉRICINE B DÉRIVÉE D'AMINES SECONDAIRES申请人:UNIV ILLINOIS公开号:WO2016112243A1公开(公告)日:2016-07-14Provided are certain urea derivatives of amphotericin B (AmB) having improved therapeutic index compared to AmB. The compounds of the invention are less toxic than AmB and are useful to treat fungal infections. In certain embodiments the urea derivative of AmB is a compound represented by formula (I) or a pharmaceutically acceptable salt thereof: wherein, independently for each occurrence: R represents methyl, ethyl, propyl, or isopropyl; R' represents methyl, ethyl, propyl, or isopropyl; or R and R', taken together with the nitrogen atom to which they are attached, represent a radical of a cyclic secondary amine. Also provided are methods for making the urea derivatives of AmB.
-
Aminopyrimidine Kinase Inhibitors申请人:Baldino Carmen M.公开号:US20110152235A1公开(公告)日:2011-06-23Disclosed are compounds, pharmaceutical compositions containing those compounds, and uses of the compounds and compositions as modulators of casein kinase 1 (e.g., CK1γ), casein kinase 2 (CK2), Pim 1, Pim2, Pim3, the TGFβ pathway, the Wnt pathway, the JAK/STAT pathway, and/or the mTOR pathway. Uses are also disclosed for the treatment or prevention of a range of therapeutic indications due at least in part to aberrant physiological activity of casein kinase 1 (e.g., CK1γ), casein kinase 2 (CK2), Pim 1, Pim2, Pim3, the TGFβ pathway, the Wnt pathway, the JAK/STAT pathway, and/or the mTOR pathway.
-
[EN] IMIDAZOPYRROLOPYRIDINE AS INHIBITORS OF THE JAK FAMILY OF KINASES<br/>[FR] IMIDAZOPYRROLOPYRIDINE EN TANT QU'INHIBITEURS DE LA FAMILLE JAK DE KINASES申请人:JANSSEN PHARMACEUTICA NV公开号:WO2018112382A1公开(公告)日:2018-06-212-((1r,4r)-4-(imidazo[4,5-d]pyrrolo[2,3-b]pyridin-1(6H)-yl)cyclohexyl)acetonitrile compounds, pharmaceutical compositions containing them, methods of making them, and methods of using them including methods for treating disease states, disorders, and conditions mediated by JAK, such as inflammatory bowel disease.2-((1r,4r)-4-(imidazo[4,5-d]pyrrolo[2,3-b]pyridin-1(6H)-yl)cyclohexyl)acetonitrile化合物,含有它们的药物组合物,制备它们的方法,以及使用它们的方法,包括用于治疗由JAK介导的疾病状态、紊乱和疾病,如炎症性肠病的方法。
-
Asymmetric Hydrogenation of α-Primary and Secondary Amino Ketones: Efficient Asymmetric Syntheses of (−)-Arbutamine and (−)-Denopamine作者:Gao Shang、Duan Liu、Scott E. Allen、Qin Yang、Xumu ZhangDOI:10.1002/chem.200700594日期:2007.9.17Two beta-receptor agonists (-)-denopamine and (-)-arbutamine were prepared in good yields and enantioselectivities by asymmetric hydrogenation of unprotected amino ketones for the first time by using Rh catalysts bearing electron-donating phosphine ligands. A series of alpha-primary and secondary amino ketones were synthesized and hydrogenated to produce various 1,2-amino alcohols in good yields and
-
CHEMICAL COMPOUNDS申请人:Deng Jianghe公开号:US20090143372A1公开(公告)日:2009-06-04The invention is directed to novel indole carboxamide derivatives. Specifically, the invention is directed to compounds according to formula I: where R1, R2, R3, U and V are defined below and to pharmaceutically acceptable salts thereof. The compounds of the invention are inhibitors of IKK2 and can be useful in the treatment of disorders associated with inappropriate IKK2 (also known as IKKβ) activity, such as rheumatoid arthritis, asthma, and COPD (chronic obstructive pulmonary disease). Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting IKK2 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
表征谱图
-
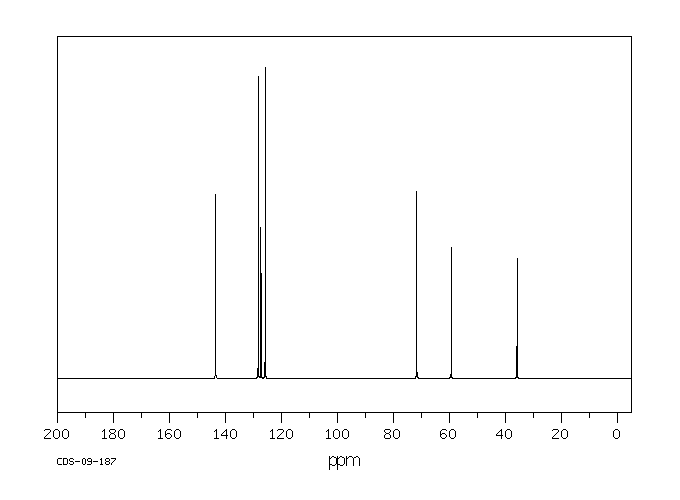
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
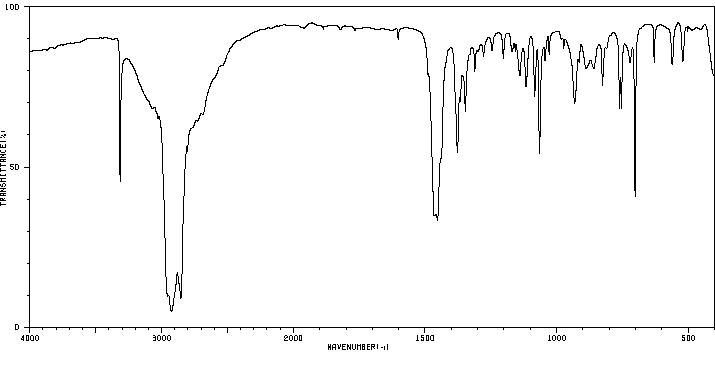
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷