2,4-喹唑啉二酮 | 86-96-4
中文名称
2,4-喹唑啉二酮
中文别名
苯并四氢嘧啶-2,4-二酮;硫化氢氨钾;2,4(1H,3H)-喹唑啉二酮;2,4-二羟基喹唑啉;亚苯甲酰基脲;2,4(1H,3H)-喹唑二酮;伸苯甲醯尿;喹唑啉-2,4-二酮
英文名称
1,2,3,4-tetrahydro-quinazoline-2,4-dione
英文别名
1H-quinazoline-2,4-dione;quinazoline-2,4(1H,3H)-dione;benzoyleneurea;quinazoline-2,4-dione;1,3-dihydroquinazoline-2,4-dione
CAS
86-96-4
化学式
C8H6N2O2
mdl
MFCD00006699
分子量
162.148
InChiKey
SDQJTWBNWQABLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:300 °C(lit.)
-
沸点:288.82°C (rough estimate)
-
密度:1.3264 (rough estimate)
-
溶解度:氢氧化铵:可溶10mg/mL
-
最大波长(λmax):217nm(EtOH)(lit.)
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:2
-
海关编码:2933990090
-
RTECS号:VA1390000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:避光,密闭保存。
SDS
| Name: | Benzoyleneurea Material Safety Data Sheet |
| Synonym: | 2,4(1H,3H)-Quinazolinedion |
| CAS: | 86-96-4 |
Synonym:2,4(1H,3H)-Quinazolinedion
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 86-96-4 | Benzoyleneurea | 201-712-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 86-96-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light pink
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 300 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6N2O2
Molecular Weight: 162.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 86-96-4: VA1390000 LD50/LC50:
CAS# 86-96-4: Oral, mouse: LD50 = >3 gm/kg.
Carcinogenicity:
Benzoyleneurea - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 86-96-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 86-96-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 86-96-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5,6,9,10-dibenzo-1,3,7-triaza-2,4,8-trioxodecane —— C15H11N3O3 281.271 3-氨基-1H-喹唑烷-2,4-二酮 3-amino-2,4(1H,3H)-quinazolinedione 30386-01-7 C8H7N3O2 177.162 2-巯基-4(3H)-喹唑酮 2-thioxo-2,3-dihydro-1H-quinazolin-4-one 13906-09-7 C8H6N2OS 178.214 2-甲基磺酰基-4(3H-)-喹唑啉酮 2-methylthioquinazolin-4(3H)-one 54855-81-1 C9H8N2OS 192.241 2-(乙硫基)喹唑啉-4(3H)-酮 2-Ethylthio-4(3H)-quinazolinone 16802-73-6 C10H10N2OS 206.268 —— 2-ethoxy-4(3H)-quinazolinone 13300-22-6 C10H10N2O2 190.202 11H-吡啶并[2,1-b]喹唑啉-11-酮 11H-pyrido[2,1-b]quinazolin-11-one 578-96-1 C12H8N2O 196.208 3-苯基-2,4(1H,3H)-喹唑啉二酮 3-phenyl-2,4-(1H,3H)-quinazolinedione 603-23-6 C14H10N2O2 238.246 —— 3-Benzalamino-(1H,3H)-quinazolin-2,4-dione 94775-15-2 C15H11N3O2 265.271 —— 3-(benzylideneamino)quinazoline-2,4(1H,3H)-dione —— C15H11N3O2 265.271 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲基喹唑啉-2,4(1h,3h)-二酮 3-methyl-2,4(1H,3H)-quinazolinedione 607-19-2 C9H8N2O2 176.175 1-甲基-2,4(1h,3h)-喹唑啉二酮 glycosmicine 604-50-2 C9H8N2O2 176.175 2,4-二羟基-6-硝基喹唑啉 6-Nitro-1H-quinazoline-2,4-dione 32618-85-2 C8H5N3O4 207.145 2-氯-4-羟基喹唑啉 2-chloro-4(3H)-quinazolinone 607-69-2 C8H5ClN2O 180.593 2-溴-4(3H)-喹唑啉酮 2-bromo-3H-quinazolin-4-one 872998-62-4 C8H5BrN2O 225.044 —— 6-benzoylquinazoline-2,4-dione 130344-84-2 C15H10N2O3 266.256 2,4-二羰基-1,2,3,4-四氢喹唑啉-6-磺酰氯 2,4-dioxo-1,2,3,4-tetrahydro-quinazoline-6-sulfonylchloride 56044-12-3 C8H5ClN2O4S 260.658 —— 1,2,3,4-tetrahydro-2,4-dioxoquinazoline-6-sulphonic acid —— C8H6N2O5S 242.212 3-乙基-2,4(1H,3H)-喹唑啉二酮 3-ethyl-1H-quinazoline-2,4-dione 2217-26-7 C10H10N2O2 190.202 1,3-二甲基-2,4-(1H,3H)-喹唑啉二酮 1,3-dimethyl-1H-quinazoline-2,4-dione 1013-01-0 C10H10N2O2 190.202 —— 3-(prop-2-yn-1-yl)quinazoline-2,4(1H,3H)-dione 1904-36-5 C11H8N2O2 200.197 —— 3-(2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl)propionaldehyde 329790-60-5 C11H10N2O3 218.212 —— 1-(methylthiomethyl)quinazoline-2,4(1H,3H)-dione —— C10H10N2O2S 222.268 —— 1-(methyloxymethyl)quinazoline-2,4(1H,3H)-dione —— C10H10N2O3 206.201 —— 2-(3-aminopropylamino)quinazolin-4(1H)-one 309976-03-2 C11H14N4O 218.258 —— 6H-quinazolino[3,2-a]quinazoline-5,12-dione 40078-06-6 C15H9N3O2 263.255 —— 1-(ethyloxymethyl)quinazoline-2,4(1H,3H)-dione —— C11H12N2O3 220.228 —— N1-(pent-4'-yn-1'-yl)quinazoline-2,4-dione —— C13H12N2O2 228.25 —— 1-(4-chlorobutyl)-2,4-dioxo-1,2,3,4-tetrahydroquinazoline 142374-71-8 C12H13ClN2O2 252.7 —— 1-(allyloxymethyl)quinazoline-2,4(1H,3H)-dione —— C12H12N2O3 232.239 —— imidazo<2,1-b>quinazolin-5-one 52998-21-7 C10H7N3O 185.185 —— 3-ethyl-1-methylquinazoline-2,4(1H,3H)-dione —— C11H12N2O2 204.228 —— 2-anilinoquinazolin-4(3H)-one 4248-15-1 C14H11N3O 237.261 —— 1-[(2-methylallyl)oxymethyl]quinazoline-2,4(1H,3H)-dione —— C13H14N2O3 246.266 —— 2-(benzylamino)-4-Quinazolinol 1791-50-0 C15H13N3O 251.288 (2,4-二氧代-3,4-二氢喹唑啉-1(2H)-基)乙酸 1,2,3,4-Tetrahydro-2,4-dioxo-chinazolin-1-yl-essigsaeure 4802-88-4 C10H8N2O4 220.185 —— 1-(2-trimethylsilyl)ethoxymethylquinazoline-2,4-dione 246264-66-4 C14H20N2O3Si 292.41 —— 3-[3-(pyridin-4-ylthio)propyl]-quinazoline-2,4(1H,3H)-dione 155967-29-6 C16H15N3O2S 313.38 3-苯基-2,4(1H,3H)-喹唑啉二酮 3-phenyl-2,4-(1H,3H)-quinazolinedione 603-23-6 C14H10N2O2 238.246 —— tetrahydro-1,2,3,4 diethyl-1,3 dioxo-2,4 quinazoline 2049-84-5 C12H14N2O2 218.255 —— 1-(benzyloxymethyl)quinazoline-2,4(1H,3H)-dione 246264-65-3 C16H14N2O3 282.299 —— 2-((4-hydroxyphenyl)amino)quinazolin-4(3H)-one 6499-59-8 C14H11N3O2 253.26 1-苯基喹唑啉-2,4-二酮 1-phenyl-2,4(1H,3H)-quinazolinedione 3282-28-8 C14H10N2O2 238.246 —— 2-(4-chlorophenylamino)quinazolin-4(3H)-one 60420-44-2 C14H10ClN3O 271.706 —— 1-((E)-cynnamyloxymethyl)quinazoline-2,4(1H,3H)-dione —— C18H16N2O3 308.337 —— 2-(3-(pyridin-4-ylmethylamino) propylamino)quinazolin-4(1H)-one 1427697-06-0 C17H19N5O 309.371 —— 1,2,3,4-Tetrahydro-2,4-dioxo-chinazolin-1-yl-essigsaeureethylester 105391-90-0 C12H12N2O4 248.238 —— 2-((4-methoxyphenyl)amino)quinazolin-4(3H)-one 1913-19-5 C15H13N3O2 267.287 —— 3-[(4-methoxyphenyl)methyl]-1H-quinazoline-2,4-dione 221539-62-4 C16H14N2O3 282.299 —— 2-((3-hydroxyphenyl)amino)quinazolin-4(3H)-one —— C14H11N3O2 253.26 —— 1-(cyclohex-3-en-1-ylmethoxymethyl)quinazoline-2,4(1H,3H)-dione —— C16H18N2O3 286.331 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:Discovery of novel potent and selective ligands for 5-HT2A receptor with quinazoline scaffold摘要:A series of compounds with quinazoline scaffold were designed, synthesized and evaluated as novel potent 5-HT2A receptor ligands. N-(4-Chlorophenyl)-2-(piperazin-1-yl)quinazolin-4-amine (5o) has a K-i value of 14.04 +/- 0.21 nM, with a selectivity more than 10,000 fold over 5-HT1A receptors (D-1 and D-2-like receptors). The functional assay showed that this compound is an antagonist to 5-HT2A receptor with an IC50 value of 1.66 mu M. (C) 2015 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2015.07.030
-
作为产物:参考文献:名称:一种制备 2,4-(1H, 3H)-喹唑啉二酮类化合物的方法摘要:本发明公开了一种制备2,4‑(1H,3H)‑喹唑啉二酮类化合物的方法,它以式(1)所示的2‑氨基苯甲氰类化合物和二氧化碳为原料,在2‑羟基吡啶型离子液体中反应得到式(II)所示的2,4‑(1H,3H)‑喹唑啉二酮类化合物,其反应式如下:。本发明的离子液体在应用于制备2,4‑(1H,3H)‑喹唑啉二酮类化合物的反应中时,反应条件较为温和,产物的分离纯化过程较为简单,产物产率高,底物适用范围广。公开号:CN112778219A
-
作为试剂:描述:、 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole tosylate 、 2,4-喹唑啉二酮 、 氢氧化钾 在 甲醇 、 2,4-喹唑啉二酮 、 crude solution 、 水 、 N,N-二甲基甲酰胺 作用下, 以 N,N-二甲基甲酰胺 、 甲醇 、 甲苯 为溶剂, 反应 4.0h, 以to give 3-[2-(2-methyl-5-nitro-1-imidazolyl)ethyl]-2,4-(1H,3H)quinazolinedione melting at about 261°-264° C.的产率得到3-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-1H-quinazoline-2,4-dione参考文献:名称:N-[2-(nitro-1-imidazolyl)ethyl]naphthal imides摘要:本文描述了一种具有硝基咪唑乙基基团作为N-取代基的咪唑类化合物。它们是抗菌和抗原虫剂。这些化合物是通过将适当的酰亚胺与氯乙基咪唑或相应的羟乙基化合物的tosylate或mesylate反应而制备的。公开号:US03997572A1
文献信息
-
Quinazolines. 4*. Acylation of quinazoline-2,4-diones with aromatic acids chlorides in the presence of ferric chloride hexahydrate作者:R. Sh. Kuryazov、Yu. R. Takhirov、D. A. Dushamov、N. S. Mukhamedov、K. K. Turgunov、Kh. M. Shakhidoyatov、B. TashkhodjaevDOI:10.1007/s10593-011-0675-6日期:2011.2The interaction of quinazoline-2,4-diones with aromatic acids chlorides in nitrobenzene has been investigated in the presence of FeCl3·6H2O. Optimum conditions for the acylation reaction have been developed, the used 4-substituted benzoyl chlorides have been put in order of relative activity, depending on the degree of their electrophilicity.
-
Synthesis and characterization of the first inhibitor of<i>N</i>-acylphosphatidylethanolamine phospholipase D (NAPE-PLD)作者:Beatrice Castellani、Eleonora Diamanti、Daniela Pizzirani、Piero Tardia、Martina Maccesi、Natalia Realini、Paola Magotti、Gianpiero Garau、Thomas Bakkum、Silvia Rivara、Marco Mor、Daniele PiomelliDOI:10.1039/c7cc07582k日期:——(NAPE-PLD) is a membrane-associated zinc enzyme that catalyzes the hydrolysis of N-acylphosphatidylethanolamines (NAPEs) into fatty acid ethanolamides (FAEs). Here, we describe the identification of the first small-molecule NAPE-PLD inhibitor, the quinazoline sulfonamide derivative 2,4-dioxo-N-[4-(4-pyridyl)phenyl]-1H-quinazoline-6-sulfonamide, ARN19874.
-
QUINAZOLINE-2,4-DIONE DERIVATIVES申请人:Hubschwerlen Christian公开号:US20140171425A1公开(公告)日:2014-06-19The invention relates to antibacterial compounds of formula (I), wherein R 1 is H, halogen, (C 1 -C 3 )alkyl or (C 1 -C 3 )alkoxy; R 2 is H, halogen, (C 1 -C 3 )alkyl, (C 1 -C 3 )alkoxy or pyrrolidin-1-yl; R 3 is H, halogen, (C 1 -C 3 )alkyl, (C 1 -C 3 )alkoxy, vinyl or 2-methoxycarbonyvinyl or R 2 and R 3 together with the two carbon atoms which bear them form a phenyl ring; R 4 is H, halogen, (C 1 -C 3 )alkyl or (C 1 -C 3 )alkoxy; and R 5 is H, (C 1 -C 3 )alkyl or cyclopropyl, or R 4 and R 5 form together a —CH 2 CH 2 CH 2 — group; A is the divalent group —CH 2 —, —CH 2 CH 2 —, #—CH(OH)CH 2 —*, #—CH 2 N(R 6 )—* and —CH 2 NHCH 2 —, wherein # indicates the point of attachment to the optionally substituted (quinazoline-2,4-dione-3-yl)methyl residue and * represents the point of attachment to the substituted (oxazolidinon-4-yl)methyl residue; R 6 is H or acetyl; Y is CH or N; and Q is O or S; and salts of such compounds.该发明涉及式(I)的抗菌化合物,其中R1为H、卤素、(C1-C3)烷基或(C1-C3)氧烷;R2为H、卤素、(C1-C3)烷基、(C1-C3)氧烷或吡咯烷-1-基;R3为H、卤素、(C1-C3)烷基、(C1-C3)氧烷、乙烯基或2-甲氧羰基乙烯基,或R2和R3与携带它们的两个碳原子一起形成苯环;R4为H、卤素、(C1-C3)烷基或(C1-C3)氧烷;R5为H、(C1-C3)烷基或环丙基,或R4和R5一起形成一个— —基团;A为二价基团—CH2—、— —、#—CH(OH) —*、#— N(R6)—*和— NH —,其中#表示可选择取代的(喹唑啉-2,4-二酮-3-基)甲基残基的连接点,*表示取代的(噁唑烷酮-4-基)甲基残基的连接点;R6为H或乙酰基;Y为CH或N;Q为O或S;以及这类化合物的盐。
-
Npy antagonists, preparation and uses申请人:Botez Iuliana公开号:US20090233910A1公开(公告)日:2009-09-17The present invention concerns novel compounds, their preparation and their uses, therapeutic uses in particular. More specifically it concerns derivative compounds having at least two aromatic cycles, their preparation and their uses, in particular in the area of human or animal health. These compounds have an affinity for the biological receptors of neuropeptide Y, NPY, present in the central and peripheral nervous systems. The compounds of the invention are preferably NPY antagonists, and more particularly antagonists of sub-type NPY Y1, and can therefore be used for the therapeutic or prophylactic treatment of any disorder involving NPY. The present invention also concerns pharmaceutical compositions containing said compounds, their preparation and their uses, as well as treatment methods using said compounds.本发明涉及新颖化合物,它们的制备和用途,特别是在治疗方面的用途。更具体地说,它涉及至少具有两个芳香环的衍生化合物,它们的制备和用途,特别是在人类或动物健康领域。这些化合物对存在于中枢和外周神经系统中的神经肽Y(NPY)的生物受体具有亲和力。本发明的化合物优选为NPY拮抗剂,更具体地说是NPY Y1亚型的拮抗剂,因此可用于治疗或预防涉及NPY的任何疾病。本发明还涉及含有所述化合物的药物组合物,其制备和用途,以及使用所述化合物的治疗方法。
-
PHARMACEUTICAL COMPOUNDS AS INHIBITORS OF CELL PROLIFERATION AND THE USE THEREOF申请人:ANDERSON MARK B.公开号:US20100068197A1公开(公告)日:2010-03-18Disclosed are compounds of Formula I effective as cytotoxic agents. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.揭示的是作为细胞毒性剂有效的I式化合物。本发明的化合物在治疗多种临床病况中是有用的,这些病况中发生异常细胞的不受控制的生长和扩散。
表征谱图
-
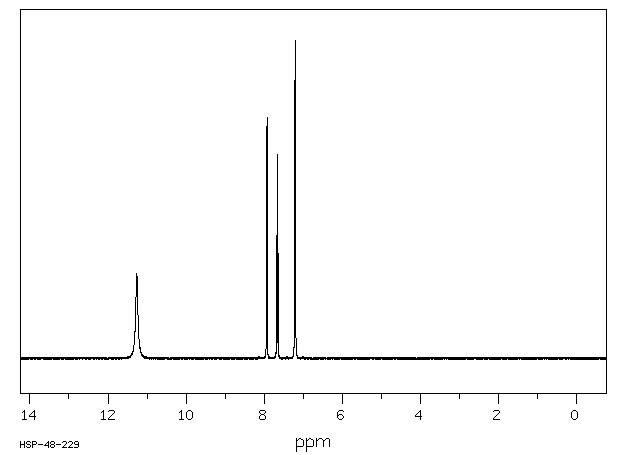
氢谱1HNMR
-
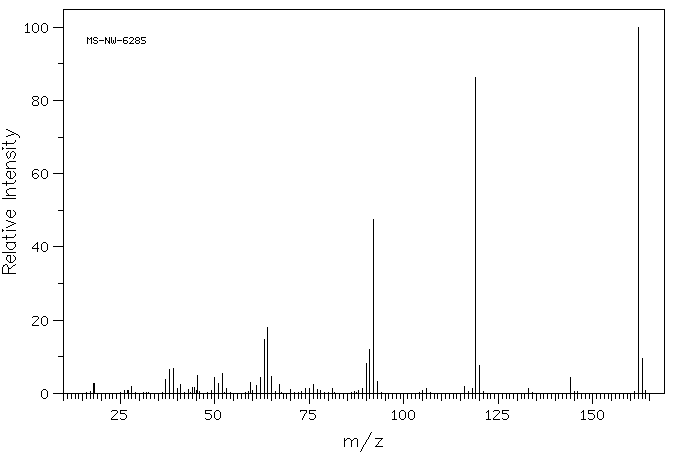
质谱MS
-
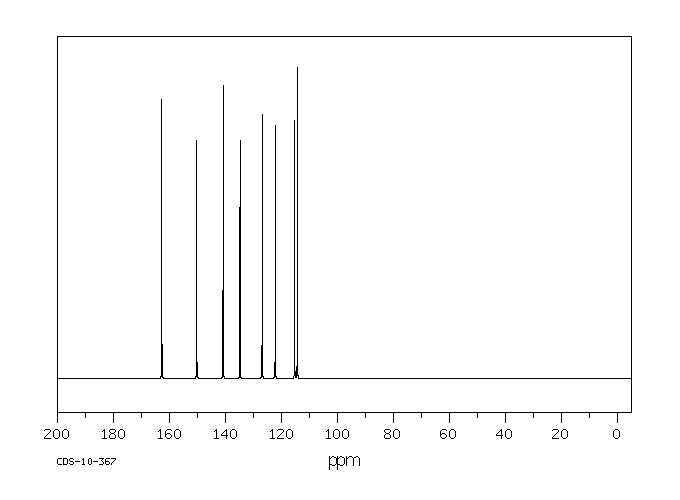
碳谱13CNMR
-
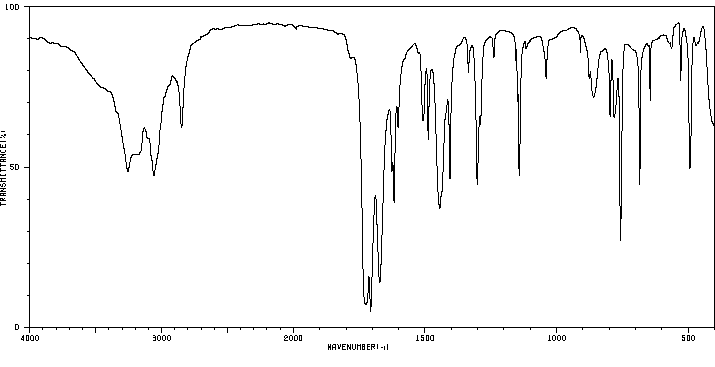
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮