1-苯基-2-吡咯烷酮 | 4641-57-0
中文名称
1-苯基-2-吡咯烷酮
中文别名
N-苯基吡咯烷酮
英文名称
1-phenylpyrrolidin-2-one
英文别名
N-phenyl-2-pyrrolidone;N-phenyl pyrrolidone;1-phenyl-2-pyrrolidinone
CAS
4641-57-0
化学式
C10H11NO
mdl
MFCD00003192
分子量
161.203
InChiKey
JMVIVASFFKKFQK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:67-69 °C (lit.)
-
沸点:123 °C/0.2 mmHg (lit.)
-
密度:1.0840 (rough estimate)
-
闪点:123°C/0.2mm
-
溶解度:可溶于氯仿(少许)、乙酸乙酯、甲醇(少许)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S37/39
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2933790090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
1-Phenyl-2-pyrrolidone Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Phenyl-2-pyrrolidone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Phenyl-2-pyrrolidone
Percent: >99.0%(GC)
CAS Number: 4641-57-0
Chemical Formula: C10H11NO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1-Phenyl-2-pyrrolidone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
1-Phenyl-2-pyrrolidone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:68°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1-Phenyl-2-pyrrolidone
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Phenyl-2-pyrrolidone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Phenyl-2-pyrrolidone
Percent: >99.0%(GC)
CAS Number: 4641-57-0
Chemical Formula: C10H11NO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1-Phenyl-2-pyrrolidone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
1-Phenyl-2-pyrrolidone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:68°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1-Phenyl-2-pyrrolidone
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
1-苯基-2-吡咯烷酮是一种吡咯烷酮化合物,属于重要的含氮杂环骨架。在天然产物中广泛存在,许多含有吡咯烷酮结构单元的天然产物和人工合成化合物都具有强大的生物活性和优异的发光性能,在新药和光电材料研发中发挥了重要作用。
制备
向10 mL史莱克管中依次加入N-苯基环己基胺(0.5 mmol,81 mg)、乙腈(5 mL)、无水醋酸铜(1 mmol,181 mg)、碘化钾(1 mmol,166 mg)和过硫酸氢钾复合盐(Oxone,615 mg,1 mmol),抽真空充氧气(1 atm)。之后将其置于80℃油浴中搅拌反应12小时。然后,加入10 mL饱和食盐水淬灭反应,用乙酸乙酯萃取三次(每次10 mL),合并有机相,用无水硫酸钠干燥。过滤后旋干,并通过硅胶柱分离(石油醚/乙酸乙酯=3/1)得到白色固体产物1-苯基-2-吡咯烷酮(47 mg,58%)。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯丁二醯亞胺 N-phenylmaleimide 83-25-0 C10H9NO2 175.187 1-(4-溴苯基)吡啶-2-酮 1-(4-bromophenyl)pyrrolidin-2-one 7661-32-7 C10H10BrNO 240.099 4-(2-氧代-1-吡咯烷)-苯甲酸 4-(2-oxopyrrolidin-1-yl)benzoic acid 36151-44-7 C11H11NO3 205.213 1-苯基-2-硫代吡咯烷-5-酮 1-phenyl-5-thioxopyrrolidin-2-one 4166-09-0 C10H9NOS 191.254 1-苯基吡咯烷 N-phenylpyrrolidine 4096-21-3 C10H13N 147.22 —— 4-hydroxy-N-phenylbutanamide 91012-01-0 C10H13NO2 179.219 —— 4-bromo-N-phenylbutanamide 7661-15-6 C10H12BrNO 242.115 4-氯-N-苯基丁酰胺 4-chloro-N-phenylbutanamide 7578-45-2 C10H12ClNO 197.664 琥珀酸一酰苯胺 N-phenyl-succinamic acid 102-14-7 C10H11NO3 193.202 —— N-phenylcyclopropanecarboxamide 2759-52-6 C10H11NO 161.203 N-苯基哌啶 N-phenyl piperidine 4096-20-2 C11H15N 161.247 1-苯基-2-氧-3-吡咯烷羧酸 2-oxo-1-phenylpyrrolidine-3-carboxylic acid 56137-52-1 C11H11NO3 205.213 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-氨基苯基)-2-吡咯烷酮 1-(4-aminophenyl)pyrrolidin-2-one 71412-08-3 C10H12N2O 176.218 1-(4-溴苯基)吡啶-2-酮 1-(4-bromophenyl)pyrrolidin-2-one 7661-32-7 C10H10BrNO 240.099 1-(4-氯苯基)-2-吡咯烷酮 1-(4-chlorophenyl)pyrrolidin-2-one 7661-33-8 C10H10ClNO 195.648 —— 3-methyl-1-phenylpyrrolidin-2-one 91596-64-4 C11H13NO 175.23 —— 4-(2-oxopyrrolidin-1-yl)benzonitrile —— C11H10N2O 186.213 1-(4-甲氧基苯基)吡咯烷-2-酮 N-(4-methoxyphenyl)-2-pyrrolidinone 30425-47-9 C11H13NO2 191.23 1-(4-硝基苯基)-2-吡咯烷酮 1-(4-nitrophenyl)pyrrolidin-2-one 13691-26-4 C10H10N2O3 206.201 —— 3-hydroxy-1-phenyl-pyrrolidin-2-one 488838-55-7 C10H11NO2 177.203 3-氯-1-苯基吡咯烷-2-酮 3-chloro-1-phenylpyrrolidin-2-one 1044674-24-9 C10H10ClNO 195.648 —— 3-methylene-1-phenylpyrrolidin-2-one 70259-90-4 C11H11NO 173.214 —— 1-(2-chlorophenyl)pyrrolidin-2-one 30425-42-4 C10H10ClNO 195.648 1-(2-溴苯基)吡咯烷-2-酮 1-(2-bromophenyl)pyrrolidin-2-one 7661-30-5 C10H10BrNO 240.099 1-(2-甲基苯基)-2-吡咯里嗪酮 1-(2-methylphenyl)pyrrolidin-2-one 24059-71-0 C11H13NO 175.23 —— 5-hydroxy-1-phenylpyrrolidin-2-one 57147-21-4 C10H11NO2 177.203 4-(2-氧-1-吡咯啉基)苯磺酰氯 4-(2-oxopyrrolidin-1-yl)benzenesulfonyl chloride 112539-09-0 C10H10ClNO3S 259.713 —— 3-(2-Hydroxyethyl)-1-phenyl-2-pyrrolidinone —— C12H15NO2 205.257 —— 1-(2,4-Dichlorophenyl)-2-pyrrolidinone 786700-38-7 C10H9Cl2NO 230.094 1-苯基吡咯烷 N-phenylpyrrolidine 4096-21-3 C10H13N 147.22 —— 3-methoxy-1-phenylpyrrolidin-2-one —— C11H13NO2 191.23 —— 1-(2-methoxyphenyl)pyrrolidin-2-one 23196-06-7 C11H13NO2 191.23 —— 3-acetyl-1-phenylpyrrolidin-2-one —— C12H13NO2 203.241 —— 1-phenyl-3-(4-phenylaminobut-1-enyl)-pyrrolidin-2-one —— C20H22N2O 306.407 —— 1-phenyl-1,5-dihydro-2H-pyrrol-2-one 55609-32-0 C10H9NO 159.188 —— 3,3-diallyl-1-phenylpyrrolidin-2-one 852339-67-4 C16H19NO 241.333 —— ethyl 2-(2-oxopyrrolidin-1-yl)phenylcarbamate 1245795-55-4 C13H16N2O3 248.282 1-苯基-2-氧-3-吡咯烷羧酸 2-oxo-1-phenylpyrrolidine-3-carboxylic acid 56137-52-1 C11H11NO3 205.213 —— 4-(2-oxopyrrolidin-1-yl)-N-phenylbenzenesulfonamide 919698-98-9 C16H16N2O3S 316.381 —— 3-(dimethylaminomethylene)-1-phenylpyrrolidin-2-one 89587-12-2 C13H16N2O 216.283 —— (3E)-3-[(dimethylamino)methylidene]-1-phenylpyrrolidin-2-one —— C13H16N2O 216.28 —— (E)-3-(2-oxopropylidene)-1-phenylpyrrolidin-2-one 129435-71-8 C13H13NO2 215.252 —— 1-(4'-bromobiphenyl-2-yl)pyrrolidin-2-one 1420040-17-0 C16H14BrNO 316.197 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:1-苯基-2-吡咯烷酮 在 dimethyl(phenyl)silyl lithium 、 碳酸氢钠 作用下, 以 四氢呋喃 为溶剂, 以25%的产率得到1-phenyl-3-(4-phenylaminobut-1-enyl)-pyrrolidin-2-one参考文献:名称:The reaction of phenyldimethylsilyllithium with N-phenylpyrrolidone摘要:苯基二甲基甲硅烷基锂与N-苯基吡咯烷酮5反应,生成已知的四环胺[2,3,3a,3b,4,5,6,11b-八氢-3aα,3bα,11bα-1-苯基-1H-二吡咯并(1,2a∶3′,2′c)喹啉及其3bβ异构体]6和7。DOI:10.1039/b210445h
-
作为产物:参考文献:名称:由t BuONO促进的N-芳基哌啶的C–C / C–N键的选择性切割和可调功能摘要:本文提出了在无金属条件下t BuONO促进的N-芳基哌啶中惰性CC -C / C-N键的选择性裂解和可调功能。具体而言,当反应在分子筛存在下在乙腈中进行时,形成了合成上有用的无环N-甲酰基腈。另一方面,当使用醇作为反应介质时,相应的反应通过机理上不同的途径提供了N-亚硝基链酯作为主要产物。DOI:10.1021/acs.orglett.9b00226
-
作为试剂:描述:4-叔丁基苯乙烯 、 重氮乙酸乙酯 在 1-苯基-2-吡咯烷酮 、 zinc trifluoromethanesulfonate 、 对甲苯磺酰胺 作用下, 以 环己烷 为溶剂, 反应 12.0h, 生成 2-(4-tert-butylphenyl)cyclopropane-1-carboxylic acid ethyl ester参考文献:名称:磺酰胺酰胺酰胺的截获:Zn(OTf)2催化合成(E)-N-磺酰基am摘要:通过用磺酰胺截留酰胺基化物,我们在此报道了磺酰胺和酰胺之间的分子间缩合反应的第一个一般实例。除甲酰胺外,该方法已成功应用于各种内酰胺和线性酰胺,从而产生了多种(E)-N-磺酰基am。DOI:10.1039/c7cc07364j
文献信息
-
Metal‐Free Synthesis of <i>N</i> ‐Aryl Amides using Organocatalytic Ring‐Opening Aminolysis of Lactones作者:Wusheng Guo、José Enrique Gómez、Luis Martínez‐Rodríguez、Nuno A. G. Bandeira、Carles Bo、Arjan W. KleijDOI:10.1002/cssc.201700415日期:2017.5.9Catalytic ring‐opening of bio‐sourced non‐strained lactones with aromatic amines can offer a straightforward, 100 % atom‐economical, and sustainable pathway towards relevant N‐aryl amide scaffolds. Herein, the first general, metal‐free, and highly efficient N‐aryl amide formation is reported from poorly reactive aromatic amines and non‐strained lactones under mild operating conditions using an organic
-
Dye–polyoxometalate coordination polymer as a photo-driven electron pump for photocatalytic radical coupling reactions作者:Zheng Ming、Tiexin Zhang、Wenming Tian、Jianing Li、Zhenhui Liu、Renhai Liu、Zhongmin Liu、Chunying DuanDOI:10.1039/d1cc04209b日期:——To alleviate diffusion-limited photoinduced electron transfer (PET) in solution, a triphenylamine-derived dye and a Keggin polyoxometalate-type electron relay were coupled into a coordination polymer to photoinduce long-lived charge-separation pairs with enough reductive/oxidative potential to pump multiple electrons unidirectionally from external electron donors to acceptors, thus furnishing photocatalytic
-
Phenyl 4-(2-oxopyrrolidin-1-yl)benzenesulfonates and phenyl 4-(2-oxopyrrolidin-1-yl)benzenesulfonamides as new antimicrotubule agents targeting the colchicine-binding site作者:Mathieu Gagné-Boulet、Chahrazed Bouzriba、Atziri Corin Chavez Alvarez、Sébastien FortinDOI:10.1016/j.ejmech.2020.113136日期:2021.3agents designated as N-phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB–SOs) and phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonamides (PIB–SAs). Our previous structure-activity relationship studies (SAR) focused on the aromatic ring B of PIB-SOs and PIB-SAs leaving the impact of the phenylimidazolidin-2-one moiety (ring A) on the binding to the colchicine-binding site (C-BS) poorly studied. Therefore我们最近设计并制备了新的有效抗微管剂家族,命名为N4-(2-氧代咪唑啉-1-基)苯磺酸苯酯(PIB-SOs)和4-(2-氧代咪唑啉-1-基)苯磺酰胺(PIB-SAs)。我们之前的结构活性关系研究(SAR)专注于PIB-SO和PIB-SA的芳香环B,而苯基咪唑啉二-2-酮部分(环A)对秋水仙碱结合位点的结合(C -BS)学习得不好。因此,本研究的目的是评估由吡咯烷-2-酮部分取代咪唑烷-2--2-酮(IMZ)的效果。为此,设计,制备了15种新的4-(2-氧杂吡咯烷-1-基)苯磺酸苯基酯(PYB-SO)和15种苯基4-(2-氧杂吡咯烷-1-基)苯磺酸酰胺(PYB-SA)衍生物,化学表征和生物学评估。PYB-SO和PYB-SA在低纳摩尔至低微摩尔范围内显示抗增殖活性(0。在人HT-1080,HT-29,M21和MCF7癌细胞系上分别为0087–8.6μM和0.056–21μM)。而且,它们阻断了G2
-
Synthesis of Lactams by Regioselective Reduction of Cyclic Dicarboximides
-
Synthesis of nitrogen heterocycles <i>via</i> α-aminoalkyl radicals generated from α-silyl secondary amines under visible light irradiation作者:Kazunari Nakajima、Mai Kitagawa、Yuya Ashida、Yoshihiro Miyake、Yoshiaki NishibayashiDOI:10.1039/c4cc03000a日期:——
We have succeeded in the visible light-mediated synthetic use of α-aminoalkyl radicals derived from α-silyl secondary amines toward addition to α,β-unsaturated carbonyl compounds. The resulting γ-aminocarbonyl compounds are converted into γ-lactams and pyrroles in a one-pot process.
表征谱图
-
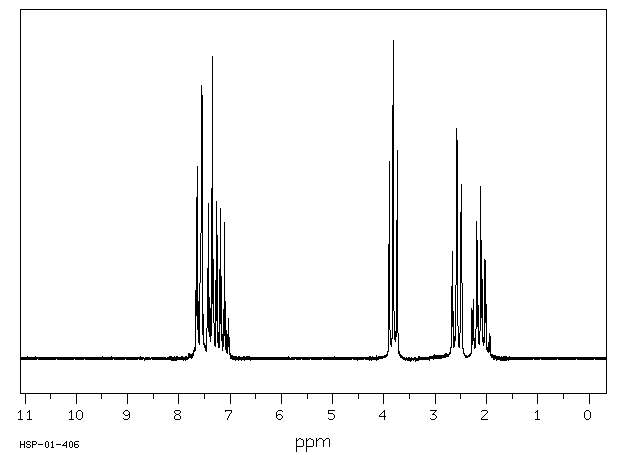
氢谱1HNMR
-
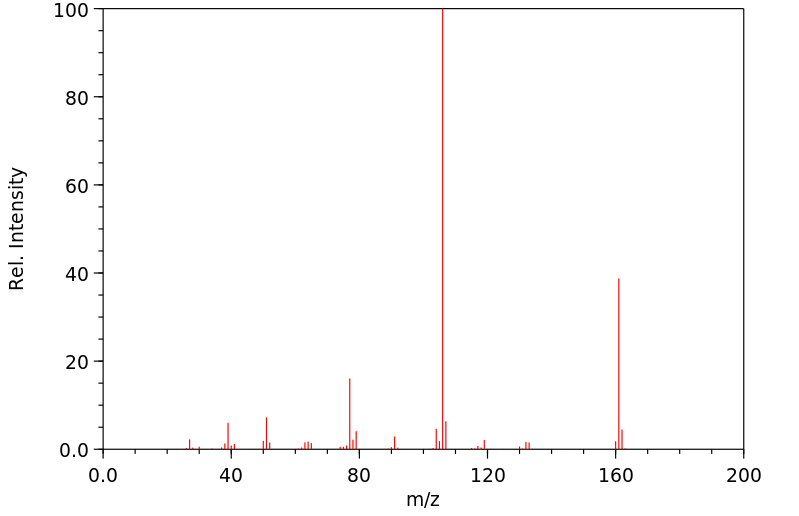
质谱MS
-
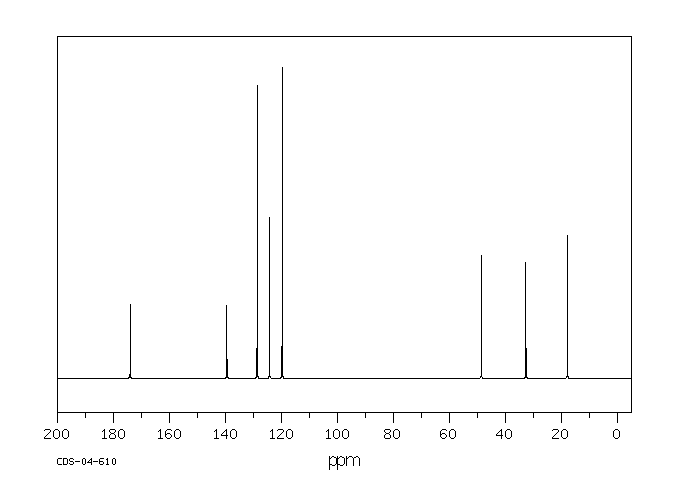
碳谱13CNMR
-
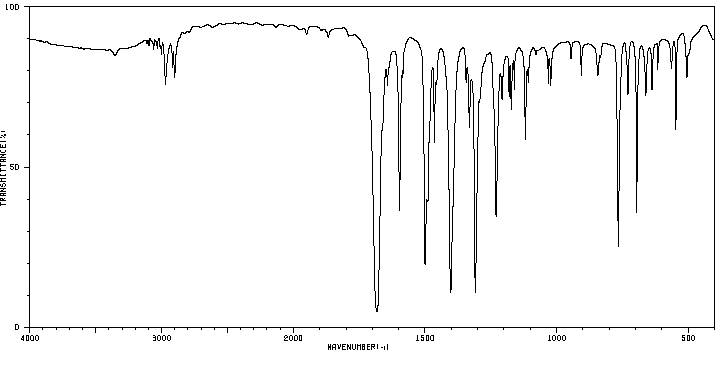
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(2R,2''R)-(-)-2,2''-联吡咯烷
麦角甾-7,22-二烯-3-基亚油酸酯
马来酰亚胺霉素
马来酰亚胺基酰肼盐酸盐
马来酰亚胺基甲基-3-马来酰亚胺基丙酸酯
马来酰亚胺丙酰基-dPEG4-NHS
马来酰亚胺-酰胺-PEG6-琥珀酰亚胺酯
马来酰亚胺-酰胺-PEG6-丙酸
马来酰亚胺-酰胺-PEG24-丙酸
马来酰亚胺-酰胺-PEG12-丙酸
马来酰亚胺-四聚乙二醇-羧酸
马来酰亚胺-四聚乙二醇-丙酸叔丁酯
马来酰亚胺-四聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-六聚乙二醇-羧酸
马来酰亚胺-六聚乙二醇-丙酸叔丁酯
马来酰亚胺-八聚乙二醇-丙酸叔丁酯
马来酰亚胺-二聚乙二醇-丙酸叔丁酯
马来酰亚胺-三(乙烯乙二醇)-丙酸
马来酰亚胺-一聚乙二醇-羧酸
马来酰亚胺-一聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-PEG3-羟基
马来酰亚胺-PEG2-胺三氟醋酸盐
马来酰亚胺-PEG2-琥珀酰亚胺酯
马来酰亚胺
频哪醇硼酸酯
顺式草酸双(-3,8-二氮杂双环[4.2.0]辛烷-8-羧酸叔丁酯)
顺式4-甲基吡咯烷酮-3-醇盐酸盐
顺式4-氟吡咯烷酮-3-醇盐酸盐
顺式3,4-二羟基吡咯烷盐酸盐
顺式3,4-二氨基吡咯烷-1-羧酸叔丁酯
顺式-二甲基 1-苄基吡咯烷-3,4-二羧酸
顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺
顺式-N-Boc-吡咯烷-3,4-二羧酸
顺式-5-苄基-2-叔丁氧羰基六氢吡咯并[3,4-c]吡咯
顺式-5-甲基-1H-六氢吡咯并[3,4-b]吡咯二盐酸盐
顺式-5-氧代六氢环戊二烯并[c]吡咯-2(1H)-羧酸叔丁酯
顺式-5-乙氧羰基-1H-六氢吡咯并[3,4-B]吡咯盐酸盐
顺式-5-(碘甲基)-4-苯基-2-吡咯烷酮
顺式-5-(碘甲基)-4-甲基-2-吡咯烷酮
顺式-4-氧代-六氢-吡咯并[3,4-C]吡咯-2-甲酸叔丁酯
顺式-3-氟-4-羟基吡咯烷-1-羧酸叔丁酯
顺式-3-氟-4-甲基吡咯烷盐酸盐
顺式-2-甲基六氢吡咯并[3,4-c]吡咯
顺式-2,5-二甲基吡咯烷
顺式-1-苄基-3,4-吡咯烷二甲酸二乙酯
顺式-1-甲基六氢吡咯并[3,4-b]吡咯
顺式-(9CI)-3,4-二乙烯-1-(三氟乙酰基)-吡咯烷
顺-八氢环戊[c]吡咯-5-酮盐酸盐
非星匹宁