环辛烷甲醛 | 6688-11-5
中文名称
环辛烷甲醛
中文别名
——
英文名称
Cyclooctyl aldehyde
英文别名
cyclooctylcarboxaldehyde;cyclooctanecarbaldehyde
CAS
6688-11-5
化学式
C9H16O
mdl
MFCD00001747
分子量
140.225
InChiKey
IGGUWVNICWZJQU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:199-200°C
-
密度:0.9271
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R10
-
危险品运输编号:UN 1989
-
海关编码:2912299000
-
安全说明:S16,S26,S36
SDS
| Name: | Cyclooctanecarboxaldehyde tech. Material Safety Data Sheet |
| Synonym: | |
| CAS: | 6688-11-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6688-11-5 | Cyclooctanecarboxaldehyde, tech. | 229-732-0 |
Risk Phrases:
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower lids.
Skin:
Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. Allow the victim to rinse his mouth and then to drink 2-4 cupfuls of water, and seek medical advice.
Inhalation:
Remove from exposure to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Autoignition Temperature: Not available.
Flash Point: 67 deg C ( 152.60 deg F) NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material, (e.g., dry sand or earth), then place into a chemical waste container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA-approved (or equivalent) full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: clear, colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Vapor Density: Not available.
Evaporation Rate: Not available.
Viscosity: Not available.
Boiling Point: 96 deg C @ 15.00mmHg
Freezing/Melting Point: 0 deg C
Decomposition Temperature: Not available.
Solubility: Not available.
Specific Gravity/Density: .9400g/cm3
Molecular Formula: C9H16O
Molecular Weight: 140.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents - reducing agents - strong bases.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6688-11-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Cyclooctanecarboxaldehyde, tech. - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
For further information, contact Fisher Scientific.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
IMO
Not regulated as a hazardous material.
IATA
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 6688-11-5:
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 6688-11-5 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 6688-11-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 环辛烷羧酸 cyclooctanecarboxylic acid 4103-15-5 C9H16O2 156.225
反应信息
-
作为反应物:描述:环辛烷甲醛 在 三乙酰氧基硼氢化钠 、 sodium hydride 作用下, 以 四氢呋喃 、 N-甲基吡咯烷酮 为溶剂, 反应 0.5h, 生成 8-cyclooctylmethyl-1-(4-fluorophenyl)-(R)-3-oxinarylmethyl-1,3,8-triazaspiro[4.5]decan-4-one参考文献:名称:[EN] HYDROXY ALKYL SUBSTITUTED 1,3,8-TRIAZASPIRO[4.5]DECAN-4-ONE DERIVATIVES USEFUL FOR THE TREATMENT OF ORL-1 RECEPTOR MEDIATED DISORDERS
[FR] DERIVES DE 1,3,8-TRIAZASPIRO[4.5]DECAN-4-ONE A SUBSTITUTION HYDROXYALKYLE UTILES POUR TRAITER DES MALADIES ASSOCIEES AU RECEPTEUR ORL-1摘要:公开号:WO2004022558A3 -
作为产物:描述:参考文献:名称:由环烷甲醛醛合成螺[环烷-1,3'-[3 H ]吲哚]。酸催化重排成环烷[ b ]吲哚摘要:螺[环烷-1,3'-[3 H ]吲哚] 2可以通过环hydr苯甲醛的苯hydr的费希尔反应从环烷甲醛1中获得。这些环化对所用的酸催化剂,溶剂和温度敏感。通过酸催化剂或通过在乙二醇中热处理2,可以将2重新排列为同源的环烷烃衍生物3。DOI:10.1002/jhet.5570220512
文献信息
-
Synthesis of sterically hindered enamides via a Ti-mediated condensation of amides with aldehydes and ketones作者:Julien Genovino、Bharat Lagu、Yaping Wang、B. Barry TouréDOI:10.1039/c2cc32538a日期:——The first TiCl(4)-mediated condensation of secondary amides with aldehydes and ketones has been achieved. The reaction proceeds at room temperature and is complete within 5 h in most cases. The optimized procedure used 5 equiv of an amine base hinting that the in situ activation of both the amide and the Lewis acid is required. The reaction affords polysubstituted (E)-enamides.已经实现了第一个TiCl(4)介导的仲酰胺与醛和酮的缩合。反应在室温下进行,在大多数情况下,反应会在5小时内完成。优化的程序使用了5当量的胺碱,这暗示着需要酰胺和路易斯酸的原位活化。反应得到多取代的(E)-酰胺。
-
FUROISOQUINOLINE DERIVATIVE AND USE THEREOF申请人:Takeda Pharmaceutical Company Limited公开号:EP1541576A1公开(公告)日:2005-06-15The present invention provides a compound represented by the formula wherein A represents (1) a bond, (2) a group represented by the formula -CRa=CRb- (Ra and Rb each represent a hydrogen atom or C1-6 alkyl) and the like; R1 represents (1) cyano or (2) an optionally esterified or amidated carboxyl group; R2 represents (1) a hydrogen atom, (2) an optionally substituted hydroxy group, (3) an optionally substituted amino group and the like; R3 and R4 each represent a hydrogen atom and the like; R5 represents a hydrogen atom and the like; R6 represents an optionally substituted hydroxy group and the like; R7and R8 each represent an optionally substituted hydrocarbon group and the like; R9 and R10 each represent (1) a hydrogen atom and the like; Y represents an optionally substituted methylene group; and n represents 0 or 1, or a salt thereof, which has an excellent phosphodiesterase IV inhibiting action.
-
[EN] FUNCTIONALISED AMINE DERIVATIVES AS IL-17 MODULATORS<br/>[FR] DÉRIVÉS D'AMINE FONCTIONNALISÉS UTILES EN TANT QUE MODULATEURS D'IL-17申请人:UCB BIOPHARMA SRL公开号:WO2020120141A1公开(公告)日:2020-06-18A series of functionalised amine derivatives of formula (I) as defined herein, being potent modulators of human IL-17 activity, are accordingly of benefit in the treatment and/or prevention of various human ailments, including inflammatory and autoimmune disorders.根据本文所定义的公式(I),一系列功能化胺衍生物作为人类IL-17活性的有效调节剂,因此在治疗和/或预防各种人类疾病,包括炎症性和自身免疫性疾病方面具有益处。
-
Novel Tricyclic Compounds申请人:Wishart Neil公开号:US20090312338A1公开(公告)日:2009-12-17The invention provides a compound of Formula (I) pharmaceutically acceptable salts, pro-drugs, biologically active metabolites, stereoisomers and isomers thereof wherein the variable are defined herein. The compounds of the invention are useful for treating immunological and oncological conditions.这项发明提供了一个符合Formula (I)的化合物,其中包括药用盐、前药、生物活性代谢物、立体异构体和同分异构体,其中变量在此处定义。该发明的化合物对治疗免疫和肿瘤疾病有用。
-
Inhibitors of c-Jun N-terminal kinases申请人:Liu Gang公开号:US20060173050A1公开(公告)日:2006-08-03The present invention relates to compounds that are inhibitors of c-jun N-terminal kinase 1, 2, or 3 (JNK1, JNK2, or JNK3), compositions containing the compounds and the use of the compounds in the prevention or treatment of disorders regulated by the activation of JNK1, JNK2 and JNK3.本发明涉及作为c-jun N-末端激酶1、2或3(JNK1、JNK2或JNK3)抑制剂的化合物,包含这些化合物的组合物以及这些化合物在预防或治疗由JNK1、JNK2和JNK3激活调控的疾病中的用途。
表征谱图
-
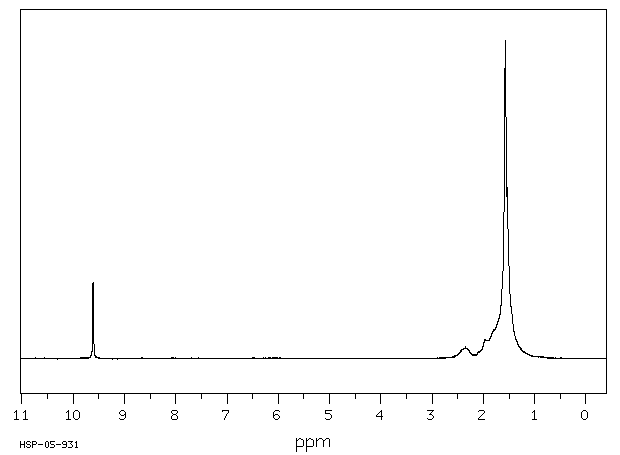
氢谱1HNMR
-
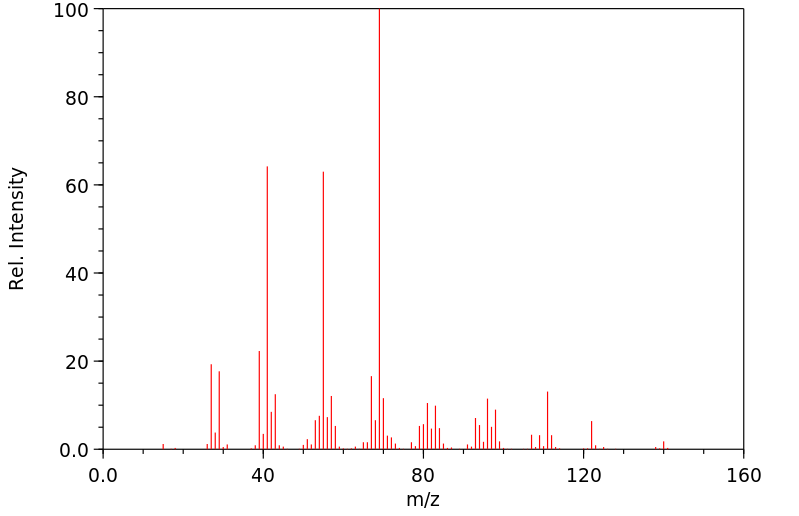
质谱MS
-
碳谱13CNMR
-
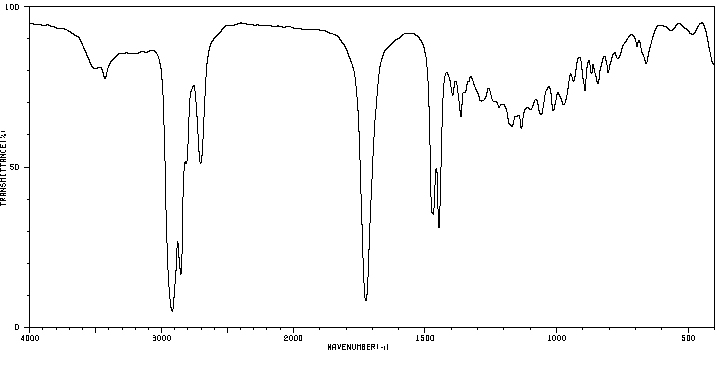
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
鲜草醛
马赛醛
顺式-环丙基-1,2-二甲醛
顺式-1-甲酰基-2-(1-己炔基)环丙烷
顺式-1,3-环己烷二甲醛
镁2-甲基丙酸盐
锶二(2-乙基-1-己醇)
锡烷,二丁基二乙氧基-
铝二异丙氧基单仲丁氧醇金属
铈(4+)四(2-甲基-2-丙醇)
過氧化二乙烷
过氧化[(1-甲基亚乙基)二-4-环己基-1-亚基]四(1,1-二甲乙基)
过氧化,3-溴丙基1,1-二甲基乙基
过氧化,1,1-二甲基乙基1-甲基乙基
表水蓼二醛
螺[4.5]癸烷-10-甲醛
聚(1-癸烯:二氧化硫)
羟基甲基叔-丁基过氧化物
甲醛-14C
甲醛-13C,D2
甲醛-13C
甲醛 [3H]
甲烷水合物
甲基6,7-二氧杂-2,3-二氮杂双环[3.2.2]壬-3,8-二烯-2-羧酸酯
甘油三酸酯过氧化物
环辛烷甲醛
环辛烷-1,4-二甲醛
环戊基甲醛
环戊基叔丁基过氧化物
环戊-3-烯-1-甲醛
环戊-2-烯-1-甲醛
环戊-2,4-二烯-1-甲醛
环戊-1,4-二烯-1-甲醛
环庚烷甲醛
环己甲醛,1,4,4-三甲基-
环己烷基甲醛
环己烷-1,3-二甲醛
环十二烷甲醛
环亚己基二[(1,1-二甲基丙基)]过氧化物
环丙甲醛
环丙-2-烯-1-甲醛
环丁基甲醛
环丁-2-烯-1-甲醛
水蓼二醛
氯代钒酸二乙酯
松油
异绒白乳菇醛
异丙醇镨(III)
异丙醇镧
异丙醇锶