呋喃-2,5-二甲酸二甲酯 | 4282-32-0
中文名称
呋喃-2,5-二甲酸二甲酯
中文别名
呋喃-2,5-二羧酸二甲酯;2,5-呋喃二甲酸二甲酯;呋喃-2,5-二甲酸甲酯
英文名称
furan-2,5-dicarboxylic acid dimethyl ester
英文别名
dimethyl 2,5-furandicarboxylate;dimethyl furan-2,5-dicarboxylate;2,5-furandicarboxylic acid dimethyl ester;FDMC;furan-2,5-dimethylcarboxylate;Furan-2,5-dicarbonsaeure-dimethylester;2,5-Furan-dicarbonsaeuredimethylester;2,5-dimethyl furan-2,5-dicarboxylate;DMFD;FDME;2,5-furandicarboxylic acid methyl ester;2,5-dimethylfuran dicarboxylate;DMFDC
CAS
4282-32-0
化学式
C8H8O5
mdl
MFCD00092317
分子量
184.149
InChiKey
UWQOPFRNDNVUOA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112°C
-
沸点:278.08°C (rough estimate)
-
密度:1.3840 (rough estimate)
-
保留指数:1346;1346
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:65.7
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险等级:IRRITANT
-
海关编码:2932190090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Dimethyl furan-2,5-dicarboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Dimethyl furan-2,5-dicarboxylate
CAS number: 4282-32-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8O5
Molecular weight: 184.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Dimethyl furan-2,5-dicarboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Dimethyl furan-2,5-dicarboxylate
CAS number: 4282-32-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8O5
Molecular weight: 184.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
呋喃-2,5-二甲酸二甲酯可用作有机合成中间体和医药中间体,主要应用于实验室研发和化工医药合成过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲氧基羰基呋喃-2-羧酸 5-(methoxycarbonyl)furan-2-carboxylic acid 6750-85-2 C7H6O5 170.122 5-甲酰基-2-呋喃甲酸甲酯 methyl 5-formylfuran-2-carboxylate 5904-71-2 C7H6O4 154.122 5-(羟基甲基)-2-糠酸甲酯 methyl 5-(hydroxymethyl)furan-2-carboxylate 36802-01-4 C7H8O4 156.138 5-甲基-2-糠酸甲酯 methyl 5-methyl-2-furancarboxylate 2527-96-0 C7H8O3 140.139 5-甲酰基-2-呋喃甲酸乙酯 ethyl 5-formyl-2-furancarboxylate 22551-91-3 C8H8O4 168.149 2,5-呋喃二甲酸 furan-2,5-dicarboxylic acid 3238-40-2 C6H4O5 156.095 5-(氯甲基)-2-糠酸甲酯 methyl 2-chloromethyl-5-furoate 2144-37-8 C7H7ClO3 174.584 2-糠酸甲酯 2-furoic acid methyl ester 611-13-2 C6H6O3 126.112 —— bis(2-ethylhexyl)furan-2,5-dicarboxylate 158099-01-5 C22H36O5 380.525 5-乙酰氧基甲基-2-呋喃醛 5-acetoxymethyl-2-furaldehyde 10551-58-3 C8H8O4 168.149 2-呋喃甲基乙酯 Ethyl 2-furoate 614-99-3 C7H8O3 140.139 5-羟甲基糠醛 5-hydroxymethyl-2-furfuraldehyde 67-47-0 C6H6O3 126.112 2-糠酸异丙酯 isopropyl 2-furoate 6270-34-4 C8H10O3 154.166 2-糠酸异丁酯 isobutyl furan-2-carboxylate 20279-53-2 C9H12O3 168.192 呋喃甲酸丁酯 furan-2-carboxylic acid butyl ester 583-33-5 C9H12O3 168.192 2-糠酸己酯 hexyl furan-2-carboxylate 39251-86-0 C11H16O3 196.246 正辛基-2-呋喃羧酸酯 octyl furan-2-carboxylate 39251-88-2 C13H20O3 224.3 5-溴-2-糠酸甲酯 methyl 5-bromo-2-furoate 2527-99-3 C6H5BrO3 205.008 糠酸(呋喃甲酸) 2-Furoic acid 88-14-2 C5H4O3 112.085 —— furan-2-carboxylic acid benzyl ester 5380-40-5 C12H10O3 202.21 —— 2-ethylhexyl furan-2-carboxylate —— C13H20O3 224.3 —— cyclobutyl furan-2-carboxylate —— C9H10O3 166.177 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-甲氧基羰基呋喃-2-羧酸 5-(methoxycarbonyl)furan-2-carboxylic acid 6750-85-2 C7H6O5 170.122 5-甲酰基-2-呋喃甲酸甲酯 methyl 5-formylfuran-2-carboxylate 5904-71-2 C7H6O4 154.122 5-(羟基甲基)-2-糠酸甲酯 methyl 5-(hydroxymethyl)furan-2-carboxylate 36802-01-4 C7H8O4 156.138 呋喃-2,5-二甲酸二乙酯 diethyl furan-2,5-dicarboxylate 53662-83-2 C10H12O5 212.202 —— 2-ethyl 5-methyl furan-2,5-dicarboxylate —— C9H10O5 198.175 —— bis(hydroxyethyl) 2,5-furandicarboxylate —— C10H12O7 244.201 —— bis(3-hydroxypropyl) 2,5-furandicarboxylate 1326203-75-1 C12H16O7 272.255 2,5-呋喃二甲酸 furan-2,5-dicarboxylic acid 3238-40-2 C6H4O5 156.095 —— 3,6,9,12,15,20-Hexaoxabicyclo[15.2.1]icosa-1(19),17-diene-2,16-dione 72638-75-6 C14H18O8 314.292 —— Bis[2-[bis(2-hydroxyethyl)amino]ethyl] furan-2,5-dicarboxylate 1041790-48-0 C18H30N2O9 418.444 —— bis(2,3-dihydroxypropyl) 2,5-furandicarboxylate —— C12H16O9 304.254 2-糠酸甲酯 2-furoic acid methyl ester 611-13-2 C6H6O3 126.112 —— bis(2-ethylhexyl)furan-2,5-dicarboxylate 158099-01-5 C22H36O5 380.525 —— methyl 5-(diethylcarbamoyl)furan-2-carboxylate —— C11H15NO4 225.244 - 1
- 2
反应信息
-
作为反应物:描述:呋喃-2,5-二甲酸二甲酯 在 C15H29MnNO3P2(1+)*Br(1-) 、 potassium tert-butylate 、 氢气 作用下, 以 1,4-二氧六环 为溶剂, 120.0 ℃ 、3.0 MPa 条件下, 反应 48.0h, 以58%的产率得到2,5-呋喃二甲醇参考文献:名称:定义的锰钳配合物催化酯加氢制醇摘要:已经开发出了第一个锰催化的酯加氢成醇的方法。Mn(CO)5 Br与[HN(CH 2 CH 2 P(Et)2)2 ]的组合产生阳离子和中性Mn PNP钳形配合物的混合物,这使得能够还原各种酯底物,包括芳族化合物和脂族酯以及二酯和内酯。值得注意的是,具有异丙基或环己基取代基的相关钳形配合物显示出非常低的活性。DOI:10.1002/anie.201607233
-
作为产物:描述:参考文献:名称:Lewkowski, Polish Journal of Chemistry, 2001, vol. 75, # 12, p. 1943 - 1946摘要:DOI:
文献信息
-
一种从糠醛生产呋喃二甲酸及其衍生物的方法申请人:华东师范大学公开号:CN111153876B公开(公告)日:2023-07-28
-
Aerobic oxidative esterification of 5-hydroxymethylfurfural to dimethyl furan-2,5-dicarboxylate by using homogeneous and heterogeneous PdCoBi/C catalysts under atmospheric oxygen作者:Feng Li、Xing-Long Li、Chuang Li、Jing Shi、Yao FuDOI:10.1039/c8gc01393d日期:——pharmaceutical intermediates. In this paper, oxidative esterification of 5-HMF has been carried out by using homogeneous and heterogeneous PdCoBi/C catalysts under atmospheric oxygen. The effect of reaction conditions on product distribution has been studied under both homogeneous and heterogeneous catalytic conditions. The highest yields of oxidative esterification products are obtained at 93% and 96% by
-
Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization作者:Juan Carlos Morales-Huerta、Antxon Martínez de Ilarduya、Sebastián Muñoz-GuerraDOI:10.1016/j.polymer.2016.02.003日期:2016.3The preparation of cyclic ethylene and butylene 2,5-furandicarboxylate oligoesters and their conversion to furan-based polyesters poly(ethylene furanoate) (PEF) and poly(butylene furanoate) (PBF) by ring-opening polymerization (ROP) are described. The cyclic oligoesters were obtained in high yields by both high dilution condensation and thermal cyclodepolymerization methods, and they consisted of mixtures描述了环状乙烯和丁烯2,5-呋喃二甲酸低聚酯的制备及其通过开环聚合(ROP)转化为呋喃基聚酯的聚呋喃乙烯酸酯(PEF)和聚呋喃丁烯酸酯(PBF)。通过高稀释缩合法和热环解聚法均能以高收率获得环状低聚酯,它们由小尺寸的混合物组成。通过半制备色谱分离出环状二聚体,三聚体和四聚体低聚酯,发现它们是熔点在140-200°C范围内的结晶化合物。混合物和单个物种的Sn(Oct)2催化的ROP得到的PEF和PBF的重均分子量为50,000至60,000 g mol -1。发现丁烯的聚合速率高于亚乙基环状低呋喃酸酯的聚合速率,并且随着循环尺寸的减小聚合速率也略有增加。通过ROP制备的PEF和PBF的热性能与通过熔融缩聚获得的这些聚酯的热性能完全一致。
-
[EN] FLOW CHEMISTRY SYNTHESIS OF ISOCYANATES<br/>[FR] SYNTHÈSE CHIMIQUE EN FLUX D'ISOCYANATES申请人:UNIV CALIFORNIA公开号:WO2021119606A1公开(公告)日:2021-06-17The disclosure provides, inter alia, safe and environmentally-friendly methods, such as flow chemistry, to synthesize isocyanates, such as methylene diphenyl diisocyanate, toluene diisocyanate, hexamethylene diisocyanate, isophorone diisocyanate, and tetramethylxylene diisocyanate.
-
[EN] FUNCTIONALIZED BIFURAN AND SYNTHESIS THEREOF<br/>[FR] BIFURANNE FONCTIONNALISÉ ET SYNTHÈSE DE CE DERNIER申请人:EXXONMOBIL CHEMICAL PATENTS INC公开号:WO2019182693A1公开(公告)日:2019-09-26A 5,5'-Di-(protected)-2,2' -bifuran: wherein each R1 is independently an unsubstituted or substituted 5- or 6-member 1,3-dioxo-2-y1 ring radical. Processes for making the bifuran include coupling 2-(protected)-furfural. Processes for using the bifuran include deprotection, functionalization, and/or polymerization to form a polyester. The polyester can be a renewable, high-performing polyester offering a combination of low cost of production, high sustainability, and excellent performance.
表征谱图
-
氢谱1HNMR
-
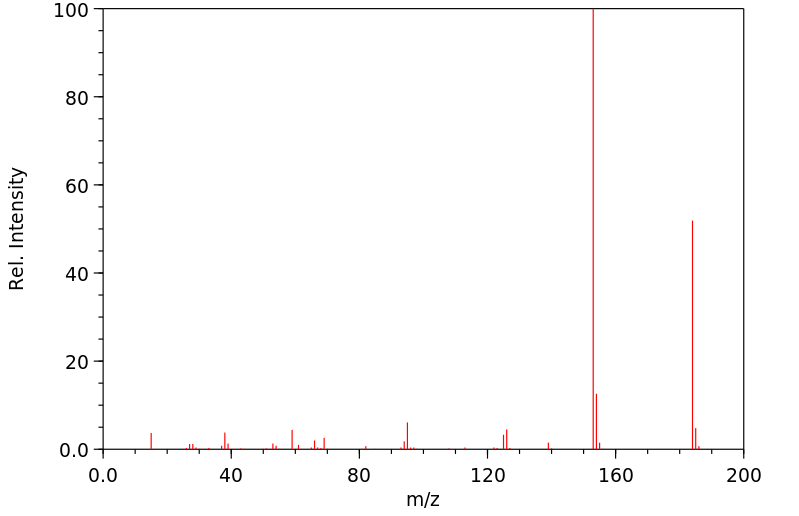
质谱MS
-
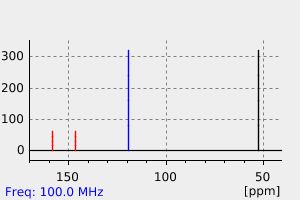
碳谱13CNMR
-
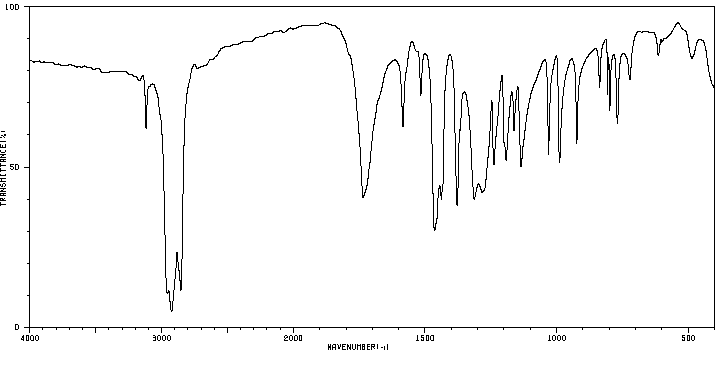
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
除草醚
锡烷,三丁基[(2-呋喃基羰基)氧代]-
醋糠硫胺
醋呋三嗪
酪氨酰-甘氨酰-色氨酰-蛋氨酰-门冬氨酰-苯基丙氨酰-甘氨酸
苯胺,N-[6-乙氧基-2,3-二(4-甲氧苯基)-4H-吡喃-4-亚基]-4-甲基-
糠酸(呋喃甲酸)
糠酸異戊酯
糠酸烯丙酯
碘化溴刚
硫代糠酸甲酯
硝基呋喃杂质
硝呋隆
硝呋醛肟标准品
硝呋达齐
硝呋美隆
硝呋维啶
硝呋立宗
硝呋甲醚
硝呋烯腙盐酸盐
硝呋烯腙
硝呋替莫
硝呋拉定
硝呋拉嗪
硝呋太尔杂质B
硝呋太尔杂质33
硝呋噻唑
硝呋吡醇
硝呋乙宗
盐酸呋喃它酮
盐酸呋喃他酮
疏呋那登
甲基7-[5-乙酰氨基-4-[(2-溴-4,6-二硝基苯基)偶氮]-2-甲氧苯基]-3-羰基-2,4,10-三氧杂-7-氮杂十一烷-11-酸酯
甲基5-溴-3-甲基-2-糠酸酯
甲基5-乙酰氨基-2-糠酸酯
甲基5-{[(氯乙酰基)氨基]甲基}-2-糠酸酯
甲基5-(甲氧基甲基)-2-甲基呋喃-3-羧酸酯
甲基5-(溴甲基)-4-(氯甲基)-2-糠酸酯
甲基5-(乙氧基甲基)-2-甲基-3-糠酸酯
甲基5-({[5-(三氟甲基)-2-吡啶基]硫代}甲基)-2-糠酸
甲基5-(4-甲酰基苯基)-2-糠酸酯
甲基5-(3-甲酰基苯基)-2-糠酸酯
甲基4-甲基-3-糠酸酯
甲基4-溴-5-甲基-2-糠酸酯
甲基4-乙酰基-5-甲基-2-糠酸酯
甲基4,6-二氯-3-(二乙基氨基)呋喃并[3,4-c]吡啶-1-羧酸酯
甲基3-羟基呋喃并[3,2-b]吡啶-2-羧酸酯
甲基3-甲酰基-2-糠酸酯
甲基3-氨基呋喃并[2,3-b]吡啶-2-羧酸酯
甲基3-氨基-5-(2-甲基-2-丙基)-2-糠酸酯