4-溴-3-硝基苯乙酮 | 18640-58-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:117-121 °C(lit.)
-
沸点:275.4℃
-
密度:1.637
-
闪点:120.4℃
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:62.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温密闭避光,通风干燥。
SDS
: 4′-Bromo-3′-nitroacetophenone
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H6BrNO3
分子式
: 244.04 g/mol
分子量
成分 浓度
1-(4-Bromo-3-nitrophenyl)ethan-1-one
-
化学文摘编号(CAS No.) 18640-58-9
EC-编号 242-469-6
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 溴化氢气
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 117 - 121 °C
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-1-(4-bromo-3-nitro-phenyl)-ethanone 22055-48-7 C8H5Br2NO3 322.941 1-溴-4-乙基-2-硝基-苯 1-bromo-4-ethyl-2-nitro-benzene 161988-89-2 C8H8BrNO2 230.061 —— 1-(4-bromo-3-nitrophenyl)ethanol 38383-27-6 C8H8BrNO3 246.06 —— 4-bromo-3-nitrostyrene 38383-33-4 C8H6BrNO2 228.045 1-(3-氨基-4-溴苯基)乙酮 5-乙酰基-2-溴苯胺 3'-amino-4'-bromoacetophenone 37148-51-9 C8H8BrNO 214.062 4-氨基-3-硝基苯乙酮 1-(4-amino-3-nitrophenyl)ethanone 1432-42-4 C8H8N2O3 180.163 4'-羟基-3'-硝基苯乙酮 4'-hydroxy-3'-nitroacetophenone 6322-56-1 C8H7NO4 181.148 —— 1-(3-nitro-4-vinyl-phenyl)-ethanone 926634-68-6 C10H9NO3 191.186 —— 1-(4-butyl-3-nitrophenyl)ethan-1-one 1565829-49-3 C12H15NO3 221.256 —— 2-Nitro-4-acetyl-diphenylamin 2043-20-1 C14H12N2O3 256.261 —— 2-Nitro-4-acetylphenylcyclopropane 39773-69-8 C11H11NO3 205.213 1-(2-硝基-(1,1’-联苯)-4-基)乙酮 4-acetyl-2-nitrobiphenyl 42771-77-7 C14H11NO3 241.246 —— 2-Nitro-4-acetyl-N-(2-dimethylamino)-ethylanilin 37429-16-6 C12H17N3O3 251.285 —— 2-Nitro-4-acetyl-N-(3-dimethylaminopropyl)-anilin 37429-18-8 C13H19N3O3 265.312 - 1
- 2
反应信息
-
作为反应物:描述:4-溴-3-硝基苯乙酮 在 盐酸 、 sodium acetate 、 亚硝酸 、 溶剂黄146 、 tin(ll) chloride 作用下, 生成 1-(1-phenyl-1H-benzotriazol-5-yl)-ethanone参考文献:名称:Borsche; Stackmann; Makaroff-Semljanski, Chemische Berichte, 1916, vol. 49, p. 2237摘要:DOI:
-
作为产物:描述:参考文献:名称:靶向丙酮酸脱氢酶激酶1的二氯苯乙酮具有提高的选择性和抗增殖活性:合成与构效关系摘要:二氯苯乙酮是一种丙酮酸脱氢酶激酶1(PDK1)抑制剂,具有次优的激酶选择性。在这里,我们报告了一系列新型二氯苯乙酮的合成和生物学评估。结构-活性关系分析(SARS)使我们能够确定3个有效的化合物,即54,55,和64,其抑制PDK1功能,激活丙酮酸脱氢酶复合物,和降低的NCI-H1975细胞的增殖。线粒体生物能量学吸附测定法表明,54,55,和64增强的癌细胞中氧化磷酸化,这可能有助于所观察到的抗增殖作用。总的来说,这些结果表明:54,55,和64可以是有前途的化合物为有效的PDK1抑制剂的开发。DOI:10.1016/j.bmcl.2018.09.026
文献信息
-
Switching Reversibility to Irreversibility in Glycogen Synthase Kinase 3 Inhibitors: Clues for Specific Design of New Compounds作者:Daniel I. Perez、Valle Palomo、Concepción Pérez、Carmen Gil、Pablo D. Dans、F. Javier Luque、Santiago Conde、Ana MartínezDOI:10.1021/jm1016279日期:2011.6.23halomethylketone moiety to reversible inhibitors turned them into irreversible inhibitors with IC50 values in the nanomolar range. Overall, the results point out that these compounds might be useful pharmacological tools to explore physiological and pathological processes related to signaling pathways regulated by GSK-3 opening new avenues for the discovery of novel GSK-3 inhibitors.
-
Rhodium(III)-Catalyzed Oxidative Annulation of Ketoximes with Sulfonamide: A Direct Approach to Indazoles作者:Ning Wang、Lingling Liu、Wentao Xu、Mengye Zhang、Zhibin Huang、Daqing Shi、Yingsheng ZhaoDOI:10.1021/acs.orglett.8b03488日期:2019.1.18A rhodium(III)-catalyzed intermolecular C–H amination of ketoxime and iodobenzene diacetate-enabled N–N bond formation in the synthesis of indazoles has been developed. A variety of functional groups were well tolerated, providing the corresponding products in moderate to good yields. Moreover, the nitro-substituted ketoximes are well compatible in this reaction, leading to the corresponding products
-
Targeting Receptor Tyrosine Kinase VEGFR-2 in Hepatocellular Cancer: Rational Design, Synthesis and Biological Evaluation of 1,2-Disubstituted Benzimidazoles作者:Heba T. Abdel-Mohsen、Mona A. Abdullaziz、Ahmed M. El Kerdawy、Fatma A. F. Ragab、Keith J. Flanagan、Abeer E. E. Mahmoud、Mamdouh M. Ali、Hoda I. El Diwani、Mathias O. SengeDOI:10.3390/molecules25040770日期:——
In this study, a novel series of 1,2-disubstituted benzo[d]imidazoles was rationally designed as VEGFR-2 inhibitors targeting hepatocellular carcinoma. Our design strategy is two-fold; it aimed first at studying the effect of replacing the 5-methylfuryl moiety of the well-known antiangiogenic 2-furylbenzimidazoles with an isopropyl moiety on the VEGFR-2 inhibitory activity and the cytotoxic activity. Our second objective was to further optimize the structures of the benzimidazole derivatives through elongation of the side chains at their one-position for the design of more potent type II-like VEGFR-2 inhibitors. The designed 1,2-disubstituted benzimidazoles demonstrated potent cytotoxic activity against the HepG2 cell line, reaching IC50 = 1.98 μM in comparison to sorafenib (IC50 = 10.99 μM). In addition, the synthesized compounds revealed promising VEGFR-2 inhibitory activity in the HepG2 cell line, e.g., compounds 17a and 6 showed 82% and 80% inhibition, respectively, in comparison to sorafenib (% inhibition = 92%). Studying the effect of 17a on the HepG2 cell cycle demonstrated that 17a arrested the cell cycle at the G2/M phase and induced a dose-dependent apoptotic effect. Molecular docking studies of the synthesized 1,2-disubstituted benzimidazoles in the VEGFR-2 active site displayed their ability to accomplish the essential hydrogen bonding and hydrophobic interactions for optimum inhibitory activity.
在这项研究中,一系列新型的1,2-二取代苯并[d]咪唑被合理设计为靶向肝细胞癌的VEGFR-2抑制剂。我们的设计策略是双重的;首先旨在研究将众所周知的抗血管生成的2-呋喃基苯并咪唑的5-甲基呋喃基团替换为异丙基基团对VEGFR-2抑制活性和细胞毒活性的影响。我们的第二个目标是通过延长它们的一位点的侧链来进一步优化苯并咪唑衍生物的结构,设计更强效的类II型VEGFR-2抑制剂。设计的1,2-二取代苯并咪唑表现出强大的细胞毒活性,对HepG2细胞系的IC50 = 1.98 μM,而索拉非尼的IC50 = 10.99 μM。此外,合成的化合物在HepG2细胞系中显示出有希望的VEGFR-2抑制活性,例如,化合物17a和6分别显示出82%和80%的抑制作用,而索拉非尼的抑制率为92%。研究17a对HepG2细胞周期的影响表明,17a在G2/M期阻滞细胞周期并诱导剂量依赖的凋亡效应。对合成的1,2-二取代苯并咪唑在VEGFR-2活性位点的分子对接研究显示它们能够完成必要的氢键和疏水相互作用,以实现最佳的抑制活性。 -
Process for the preparation of tricyclic amino alcohol derivatives申请人:ASAHI KASEI KABUSHIKI KAISHA公开号:US20030225289A1公开(公告)日:2003-12-04A process for the preparation of tricyclic amino alcohol derivatives including 2-[N-[2-(9H-carbazol-2-yloxy)ethyl]]amino-1-[(3-methylsulfonylamino)phenyl]ethanol useful in the treatment of diabetes, obesity, hyperlipidemia and so on; and intermediates as represented by formula (5) or (6) or the like useful in the preparation, wherein R11 is hydrogen or the like; and *1 represents an asymmetric carbon atom. 2-Halo-1-(3-nitrophenyl)ethanone derivatives and 1-(3-nitrophenyl)oxirane derivatives, which are intermediates for the preparation of tricyclic amino alcohol derivatives, are easy of purification, and particularly optically active 1-(3-nitrophenyl)oxirane derivatives are effective in enhancing the optical purities of the final products.
-
A Diastereoselective Total Synthesis of <i>trans</i>-Trikentrin A: A Ring Contraction Approach作者:Luiz F. Silva、Marcus V. CraveiroDOI:10.1021/ol8023105日期:2008.12.4obtain the polyalkylated indole (+/-)-trans-trikentrin A was developed. The synthesis of this natural alkaloid features a thallium(III)-mediated ring contraction reaction to obtain the trans-1,3-disubstituted five-membered ring in a diastereoselective manner. Thallium(III) is chemoselective in this rearrangement, reacting with the olefin without oxidation of the indole moiety. Other key transformations
表征谱图
-
氢谱1HNMR
-
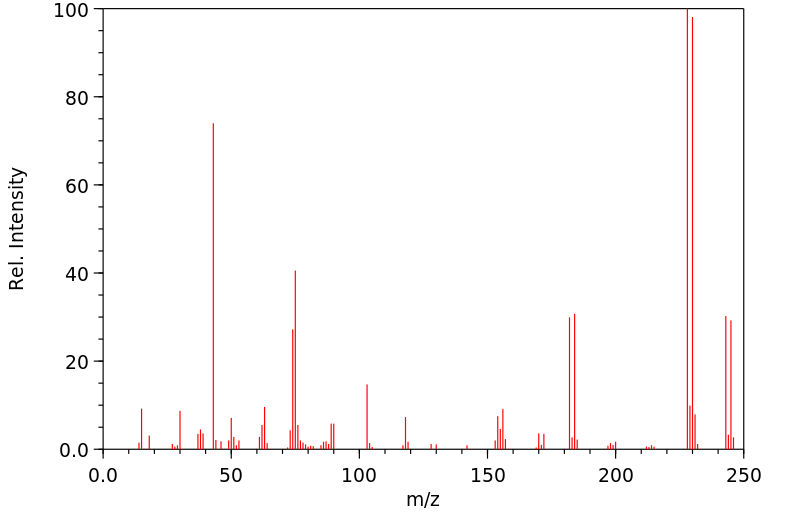
质谱MS
-
碳谱13CNMR
-
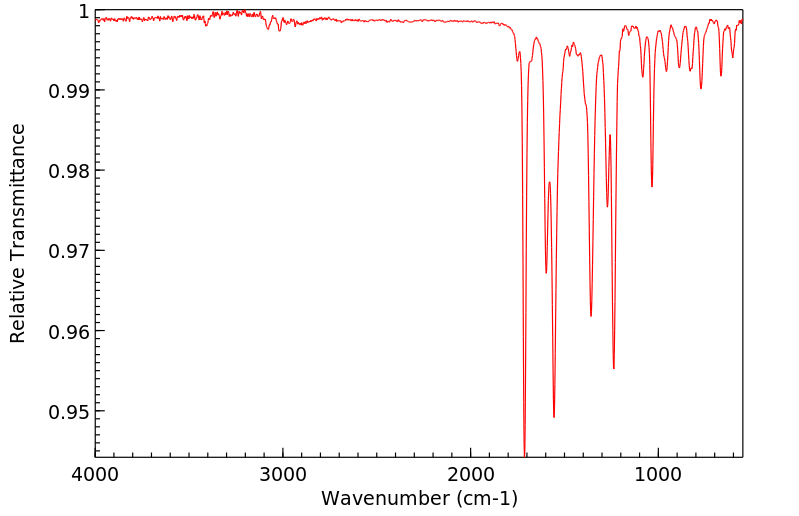
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息