硝酸异丙酯 | 1712-64-7
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-99.99°C
-
沸点:101-102 °C(lit.)
-
密度:1.040 g/mL at 20 °C(lit.)
-
闪点:55 °F
-
介电常数:11.5(19℃)
-
LogP:1.66
-
物理描述:Isopropyl nitrate appears as a clear colorless liquid with a pleasant odor. May spontaneously decompose and explode under prolonged exposure to fire or heat. Denser than water and insoluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion.
-
溶解度:0.03 M
-
蒸汽压力:34.10 mmHg
-
亨利常数:0.00 atm-m3/mole
-
大气OH速率常数:4.90e-13 cm3/molecule*sec
-
保留指数:663.8;664;664;693
-
稳定性/保质期:
- 稳定性 稳定。
- 禁配物 强还原剂、强酸、活性金属粉末。
- 避免接触的条件 受热。
- 聚合危害 不聚合。
- 分解产物 氮氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3.1
-
危险品标志:O,F,Xn
-
安全说明:S16,S17,S33,S7/9
-
危险类别码:R5,R8,R11,R20
-
WGK Germany:3
-
危险品运输编号:UN 1222 3/PG 2
-
海关编码:2920909090
-
危险类别:3.1
-
RTECS号:QU8930000
-
包装等级:II
-
储存条件:储存时应注意以下事项: - 储存在通风良好且低温的库房内,库温不宜超过37℃。 - 远离火源和热源。 - 保持容器密封。 - 应与还原剂、酸类及活性金属粉末分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用可能产生火花的机械设备和工具。 - 储存区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
用途
该物质主要用于医药中间体。此外,它也用作汽车燃料添加剂及喷气式飞机辅助推进剂,并且在某些情况下还用作溶剂。
生产方法
通过异丙醇与硝酸在尿素存在下进行直接酯化反应制得硝酸异丙酯。随后经过分离、水洗、中和、干燥以及精馏等步骤,最终得到成品。
类别
易燃液体
毒性分级
中毒
急性毒性
吸入:小鼠的致死浓度为65,000毫克/立方米(2小时)
爆炸物危险特性
受热时可能发生爆炸
可燃性危险特性
遇明火、高温或氧化剂会燃烧,产生有毒氮氧化物烟雾
储运特性
应存放于通风良好且低温干燥的库房中,并与氧化剂分开储存
职业标准
时间加权平均容许浓度(TWA)为45毫克/立方米;短时间接触容许浓度(STEL)为78毫克/立方米
反应信息
-
作为反应物:参考文献:名称:C 3 C 5硝酸盐的紫外线吸收截面和光解产物的温度依赖性摘要:腔衰荡光谱法已被用来测量1-丙基,2-丙基,1-丁基和1-戊基硝酸盐的紫外吸收截面随温度在265至340 nm之间的变化。吸收截面随温度从238K增加到298K。研究了这些C 3 C 5烷基硝酸盐在308 nm处的光解离;唯一的碎裂通道是RO-NO 2键断裂形成RO + NO 2。NO 2的产率为1.0±0.1,与温度无关。计算了C 3 C 5烷基硝酸盐的对流层光解速率。DOI:10.1016/s0009-2614(97)01011-7
-
作为产物:描述:参考文献:名称:SOLVOLYSIS IN HYDROGEN AND DEUTERIUM OXIDE: II. STRONGLY SOLVATED SUBSTRATES摘要:在轻水和重水中,给出了一系列极性底物的溶解速率,从中可以获得速率比[公式:见文本]。研究的化合物包括甲基、o-甲基、p-甲基、p-甲氧基和p-氟基苯磺酸酯,烷基甲磺酸酯的α-和β-甲基化系列,甲基和异丙基硝酸酯,甲基磷酸酯和硫酸酯,甲烷和苯磺酰氯,以及叔丁基二甲基磺酸盐。尽管氧阴离子酯的反应性存在很大变化,但速率比几乎保持在0.9附近。氯化物的值为0.63,磺酸盐的值为0.95。速率比未能反映由于烷基基团和氧阴离子变化所需的机械变化,这归因于初始状态溶剂化的结构和过渡态中小电荷发展易于饱和影响比率的结构变化。DOI:10.1139/v57-175
-
作为试剂:参考文献:名称:微波增强的Friedländer合成法可快速组装卤化喹啉,并具有针对耐药性和耐受性细菌的抗菌和生物膜清除活性。摘要:本文中,我们公开了无催化剂和无保护基团的微波增强Friedländer合成技术的开发,该技术可实现多种8-羟基喹啉的一步聚合收敛,与传统油浴加热相比,反应产率大大提高(从34%提高到至72%)。这种快速合成使得发现了对MRSA,MRSE和VRE具有活性的新型生物膜消除卤代喹啉(MBEC = 1.0-23.5μM)。这些小分子通过独立于膜裂解的机制表现出活性,进一步证明了其作为针对持久性生物膜相关感染的临床有用治疗选择的潜力。DOI:10.1039/c6md00381h
文献信息
-
Tricyclic quinoxalinediones申请人:Sumitomo Pharmaceuticals Company, Limited公开号:US05616586A1公开(公告)日:1997-04-01Tricyclic quinoxalinediones of the formula: ##STR1## wherein X is alkyl, halogen, cyano, trifluoromethyl, nitro, hydroxy, amino, etc.; R.sup.1 is H, etc.; R.sup.2 is H, alkyl, cycloalkyl, alkenyl, alkynyl, cycloalkylalkyl, arylalkyl, substituted arylalkyl, aryl, or substituted aryl; W is H, CO.sub.2 R.sup.3, CO.sub.2 Y, CONR.sup.3 R.sup.4, CONR.sup.3 Y, CON(OR.sup.3)R.sup.4, COR.sup.3, CN, tetrazolyl, or substituted alkyl; R.sup.3 and R.sup.4 independently are H, alkyl, cycloalkyl, alkenyl, alkynyl, etc.; Y is mono-substituted alkyl or di-substituted alkyl; and n is an integer 0 or 1, or a pharmaceutically acceptable salt thereof, which are useful as a selective antagonist of glutamate receptor for the treatment or prevention of various diseases in animals including human being, for example, minimizing damage of central nervous system induced by ischaemic or hypoxylic conditions, treatment and/or prevention of neurodegenerative disorders, and further analgesics, antidepressants, anxiolitics, and anti-schizophrenics.Tricyclic quinoxalinediones的中文翻译:其中X是烷基、卤素、氰基、三氟甲基、硝基、羟基、氨基等;R.sup.1是H等;R.sup.2是H、烷基、环烷基、烯基、炔基、环烷基烷基、芳基烷基、取代芳基烷基、芳基或取代芳基;W是H、CO.sub.2 R.sup.3、CO.sub.2 Y、CONR.sup.3 R.sup.4、CONR.sup.3 Y、CON(OR.sup.3)R.sup.4、COR.sup.3、CN、四唑基或取代烷基;R.sup.3和R.sup.4独立地是H、烷基、环烷基、烯基、炔基等;Y是单取代烷基或双取代烷基;n是整数0或1,或其药学上可接受的盐,可用作选择性谷氨酸受体拮抗剂,用于治疗或预防动物包括人类的各种疾病,例如,减少由缺血或低氧条件引起的中枢神经系统损伤,治疗和/或预防神经退行性疾病,以及进一步的镇痛剂、抗抑郁药、抗焦虑药和抗精神分裂症药物。
-
Manufacture of nitric acid esters申请人:SHARPLES CHEMICALS INC公开号:US02294849A1公开(公告)日:1942-09-01
540,050. Alkyl nitrates. STEVENS, A. H. (Sharples Solvents Corporation). March 29, 1940, No. 5722. Drawings to Specification. [Class 2 (iii)] Alkyl nitrates are produced by reacting in alcohol with nitric acid of 35-68 per cent. strength under a pressure of 20-650 mm., the nitrate being removed by azeotropicdistillation, water (or in the case of ethyl or methyl nitrate, the ester) being returned from the distillate or otherwise to the reaction vessel to prevent accumulation of ester or water. The invention is applicable to the esterification of dihydric alcohols. Urea is preferably added during the process, and the water to be returned may be added in the form of solvent or diluent of the nitric acid, urea or alcohol. Examples 1-11 describe the esterification of n-butyl, 2-chlorethyl, mixed amyl, secondary amyl, iso-amyl, n-amyl, n-hexyl, secondary hexyl, 2-ethoxyethyl, cyclohexyl, and octyl nitrates, using the reagents in the liquid phase. The alcohol may be supplied in the liquid or vapour phase. Another example describes the production of isopropyl nitrate, the alcohol being supplied in the form of vapour above the surface of the nitric acid. Specification 379,312 is referred to.
540,050. 烷基硝酸酯。史蒂文斯,A.H.(夏普尔斯溶剂公司)。1940年3月29日,编号5722。按照规范进行绘图。[2类(iii)] 烷基硝酸酯是通过在35-68%浓度的硝酸中,与醇在20-650毫米压力下反应制得,通过共沸蒸馏去除硝酸酯,从蒸馏液中返回水(或在乙酸乙酯或甲酸乙酯的情况下,返回酯)到反应容器中,以防止酯或水的积累。该发明适用于二元醇的酯化。在过程中最好添加尿素,并且要返回的水可以以溶剂或稀释剂的形式添加到硝酸、尿素或醇中。示例1-11描述了在液相中使用试剂对正丁基、2-氯乙基、混合戊基、次戊基、异戊基、正戊基、正己基、次己基、2-乙氧基乙基、环己基和辛基硝酸酯进行酯化。醇可以以液态或气态形式提供。另一个示例描述了异丙基硝酸酯的生产,醇以气态形式供应在硝酸表面之上。参考规范379,312。 -
Hydroxyl-radical-initiated oxidation of isobutyl isopropyl ether under laboratory conditions related to the troposphere Product studies and proposed mechanism作者:Konrad Stemmler、Wolfgang Mengon、J. Alistair KerrDOI:10.1039/a701766i日期:——The products formed by the hydroxyl-radical-initiated oxidation of the model ether, isobutyl isopropyl ether [(CH 3 ) 2 CHCH 2 OCH(CH 3 ) 2 ], have been investigated by irradiating synthetic air mixtures containing the substrate, methyl nitrite, and nitric oxide at ppm levels in a Teflon bag reactor at room temperature. The decay of reactant and formation of products were monitored by gas chromatography, mass spectrometry and by HPLC. The molar yields of the major products (mol of product formed/mol of isobutyl isopropyl ether consumed) were as follows: acetone, 0.56 ± 0.04; isopropyl formate, 0.48 ± 0.03; isobutyl acetate, 0.28 ± 0.02; 2-hydroxy-2-methylpropyl acetate [CH 3 C(O)OCH 2 C(OH)(CH 3 ) 2 ], 0.25 ± 0.1. The molar yields of the minor products were as follows: isobutyraldehyde, 0.06 ± 0.05; isopropyl nitrate, 0.09 ± 0.06; 1,1,4-trimethyl-3-oxapentyl nitrate [(CH 3 ) 2 CHOCH 2 C(CH 3 ) 2 (ONO 2 )], 0.07 ± 0.02; isopropyl isobutyrate [(CH 3 ) 2 CHC(O)OCH(CH 3 ) 2 ] ca. 0.01; and isobutyl formate, ca. 0.01. The major products are explained by a mechanism involving initial OH attack at the –CH– and –CH 2 – groups in the alkyl side chains of the ether followed by the subsequent reactions of the resulting carbon-centred, organic peroxy, and organic oxy radicals. The observed products, in conjunction with the proposed reaction pathways, account for a total yield of about 1.15, indicating that all the main routes are accounted for in the degradation of this ether. The major reaction pathways of the three principal organic oxy radicals are summarised as follows (percentage of overall reaction in brackets):(CH3)2C(O˙)OCH2CH(CH3)2 → CH3C(O)OCH2CH(CH3)2]C˙H3 (28%)(CH3)2CHOCH(O˙)CH(CH3)2 → (CH3)2CHOC(O)H]C˙H(CH3)2 (⩽48%)(CH3)2CHOCH2C(O˙)(CH3)2 → (CH3)2C˙OCH2C(OH)(CH3)2 (25%)This study supports the finding that organic oxy radicals generated from ethers and containing the structure RCH(O˙)OR undergo mainly decomposition by C–C bond cleavage, whereas those oxy radicals with the structure RCH(O˙)CH 2 OR undergo preferential 1,5-H-atom transfer isomerisation reactions. The following rate coefficients (10 −12 cm 3 molecule −1 s −1 ) at room temperature for the reactions of OH radicals with the reactant and products have been determined by the relative rate technique: isobutyl isopropyl ether, 19.5 ± 0.4; isobutyl acetate, 6.0 ± 0.5; isobutyraldehyde, 25.8 ± 0.7; isopropyl formate, 2.1 ± 0.1; isopropyl isobutyrate, 6.5 ± 0.4; 1,1,4-trimethyl-3-oxapentyl nitrate, 16.5 ± 0.7; and 2-hydroxy-2-methylpropyl acetate, 9.5 ± 1.6.通过将含有底物、亚硝酸甲酯和硝酸氧化物的合成空气混合物在室温下的特氟龙袋反应器中以ppm水平辐照,研究了异丁基异丙基醚[(CH 3 ) 2 CHCH 2 OCH(CH 3 ) 2 ] 由羟基自由基引发的氧化反应形成的产品。通过气相色谱、质谱和高效液相色谱监测反应物的衰减和产品的形成。主要产品的摩尔产率(形成的产品摩尔数/消耗的异丁基异丙基醚摩尔数)如下:丙酮,0.56 ± 0.04;异丙基甲酸酯,0.48 ± 0.03;异丁基乙酸酯,0.28 ± 0.02;2-羟基-2-甲基丙基乙酸酯[CH 3 C(O)OCH 2 C(OH)(CH 3 ) 2 ],0.25 ± 0.1。次要产品的摩尔产率如下:异丁醛,0.06 ± 0.05;异丙基硝酸酯,0.09 ± 0.06;1,1,4-三甲基-3-氧代戊基硝酸酯[(CH 3 ) 2 CHOCH 2 C(CH 3 ) 2 (ONO 2 )],0.07 ± 0.02;异丙基异丁酸酯[(CH 3 ) 2 CHC(O)OCH(CH 3 ) 2 ],约0.01;异丁基甲酸酯,约0.01。主要产品可通过以下机制解释:羟基初始攻击醚的烷基侧链中的−CH−和−CH 2 –基团,随后由产生的碳中心、有机过氧和有机氧自由基进行后续反应。观测到的产品与提出的反应途径一起,总产率约为1.15,表明在降解此醚的所有主要途径都得到了解释。三个主要有机氧自由基的主要反应途径总结如下(括号内为总体反应的百分比):(CH3)2C(O˙)OCH2CH( )2 → C(O)OCH2CH( )2]C˙H3 (28%),( )2CHOCH(O˙)CH( )2 → ( )2CHOC(O)H]C˙H( )2 (⩽48%),( )2CHOCH2C(O˙)( )2 → ( )2C˙OCH2C(OH)( )2 (25%)。本研究支持了以下发现:来自醚的有机氧自由基且含有结构RCH(O˙)OR的主要是通过C–C键断裂分解,而那些具有结构RCH(O˙)CH 2 OR的有机氧自由基则优先进行1,5-H-原子转移异构化反应。通过相对速率技术确定了以下反应的速率系数(10 −12 cm 3 molecule −1 s −1 )在室温下为:异丁基异丙基醚,19.5 ± 0.4;异丁基乙酸酯,6.0 ± 0.5;异丁醛,25.8 ± 0.7;异丙基甲酸酯,2.1 ± 0.1;异丙基异丁酸酯,6.5 ± 0.4;1,1,4-三甲基-3-氧代戊基硝酸酯,16.5 ± 0.7;2-羟基-2-甲基丙基乙酸酯,9.5 ± 1.6。
-
Severe Prolonged Tacrolimus Overdose with Minimal Consequences作者:Laura L. Hardwick、Thomas D. BatiukDOI:10.1592/phco.22.12.1063.33604日期:2002.8A 59-year-old man inadvertently received a 10-fold increase in his twice-daily oral dose of tacrolimus 1 mg that resulted in trough blood levels above 90 ng/ml for over a week. The patient had end-stage renal disease secondary to diabetes mellitus and had received a kidney transplant from his daughter 3 months earlier. Despite the numerous adverse effects commonly reported with tacrolimus, such as mild nephrotoxicity, nausea, tremors, and elevated liver enzyme levels, our patient's acute but prolonged overdose resulted in minimal signs and symptoms of toxicity. Nevertheless, education regarding the importance of accurate dosing, close monitoring, potential drug interactions, and the various capsule colors should be provided to all patients who receive tacrolimus, as well as their physicians, nurses, and pharmacists, in order to prevent as many errors as possible.
-
2,3-dioxo-1,2,3,4-tetrahydro-quinoxalinyl derivatives申请人:Novartis AG公开号:US06080743A1公开(公告)日:2000-06-272,3-Dioxo-1,2,3,4-tetrahydro-quinoxalinyl derivatives of formula (I), ##STR1## wherein one of the radicals R.sub.1, and R.sub.2 is a group R.sub.5 and the other is a group of formula --CH(R.sub.6)--alk--R.sub.7 (Ia), --alk--CH(R.sub.6 -R.sub.7 (Ib), --alk--N(R.sub.8)--X--R.sub.7 (Ic), --alk--N.sup.+ (R.sub.8)(R.sup.9)--X--R.sub.7 A.sup.- (Id), --alk--O--X--R.sub.7 (Ie) or --alk--S--X--R.sub.7 (If), R.sub.3, R.sub.4 and R.sub.5 are each independently of the others hydrogen, lower alkyl, halogen, trifluoromethyl, cyano or nitro, R.sub.6 is unsubstituted or lower alkylated and/or lower alkanoylated amino, R.sub.7 is hydrogen; an aliphatic, cycloaliphatic or heterocycloaliphatic radical; cyano; acyl derived from carbonic acid or from a semiester or semiamide of carbonic acid, from sulfuric acid or from an aliphatic or aromatic sulfonic acid or from phosphoric acid or from a phosphonic acid ester; amino that is unsubtituted or aliphatically or araliphatically substituted and/or substituted by aliphatic, araliphatic or aromatic acyl; or an aromatic or heteroaromatic radical, R.sub.8 is hydrogen; an aliphatic or araliphatic radical; or acyl derived from an aliphatic or araliphatic carboxylic acid or from an aliphatic or araliphatic semiester of carbonic acid, or R.sub.7 and R.sub.8, together with X and the nitrogen atom bonding R.sub.8 and X, form an unsubstitued or substituted mono- or di-azaxycloalkyl, azoxacycloalkyl, azathiacycloalkyl or optionally oxidised thiacycloalkyl radical bonded via a nitrogen atom, or an unsubstituted or substituted, optionally partially hxdrogenated aryl or heteroaryl radical, R.sub.9 is an aliphatic or araliphatic radical, or R.sub.7, R.sub.8 and R.sub.9 together with X and the nitrogen atom bonding R.sub.8, R.sub.9 and X, form an unsubstituted or substituted quaternary heteroaryl radical bonded via the quaternary nitrogen atom, with A.sup.- being the anion of a protonic acid, alk is lower alkylene, and X (unless, together with R.sub.7 and R.sub.8 and the nitrogen atom bonding R.sub.8 and X or together with the nitrogen atom bonding R.sub.8, R.sub.9 and X, it forms part of one of the mentioned ring systems) is a divalent aliphatic, cycloaliphatic or araliphatic radical or a direct bond, and the pharmaceutically acceptable salts thereof can be used in the preparation of a medicament for the treatment of pathological conditions that are responsive to blocking of AMPA, kainate and/or glycine binding sites of the NMDA receptor.公式(I)的2,3-二氧代-1,2,3,4-四氢喹啉基衍生物,其中R.sub.1和R.sub.2中的一个是R.sub.5基团,另一个是--CH(R.sub.6)--alk--R.sub.7 (Ia)、--alk--CH(R.sub.6)--R.sub.7 (Ib)、--alk--N(R.sub.8)--X--R.sub.7 (Ic)、--alk--N.sup.+ (R.sub.8)(R.sup.9)--X--R.sub.7 A.sup.- (Id)、--alk--O--X--R.sub.7 (Ie)或--alk--S--X--R.sub.7 (If)的公式基团;R.sub.3、R.sub.4和R.sub.5各自独立地是氢、低碳基、卤素、三氟甲基、氰基或硝基;R.sub.6是未取代或低碳基化和/或低脂肪酰化的氨基;R.sub.7是氢;一种脂肪、环脂或杂环脂基;氰基;从碳酸或碳酸半酯或半酰胺、硫酸或脂肪或芳香磺酸、磷酸或膦酸酯衍生的酰基;未取代或烷基或芳基取代和/或烷基、芳基或芳香酰基取代的氨基;或芳香或杂环芳基,R.sub.8是氢;一种脂肪或芳基基团;或从脂肪或芳香羧酸或从碳酸的脂肪或芳香半酯衍生的酰基,或R.sub.7和R.sub.8连同X和与R.sub.8和X连接的氮原子形成未取代或取代的单环或二环氮杂环脂、氧杂环脂、硫杂环脂或可选择氧化的硫杂环脂基,通过氮原子连接,或未取代或取代的、可选择部分氢化的芳基或杂芳基基团,R.sub.9是一种脂肪或芳基基团,或R.sub.7、R.sub.8和R.sub.9连同X和连接R.sub.8、R.sub.9和X的氮原子形成通过季铵氮原子连接的未取代或取代的杂芳基基团,A.sup.-是质子酸的阴离子,alk是低碳烷基,X(除非与R.sub.7和R.sub.8以及连接R.sub.8和X的氮原子或与连接R.sub.8、R.sub.9和X的氮原子形成所述环系统之一的一部分)是二价的脂肪、环脂或芳基基团或直接键,其药学上可接受的盐可用于制备用于治疗对AMPA、kainate和/或甘氨酸-NMDA受体结合位点阻塞敏感的病理条件的药物。
表征谱图
-
氢谱1HNMR
-
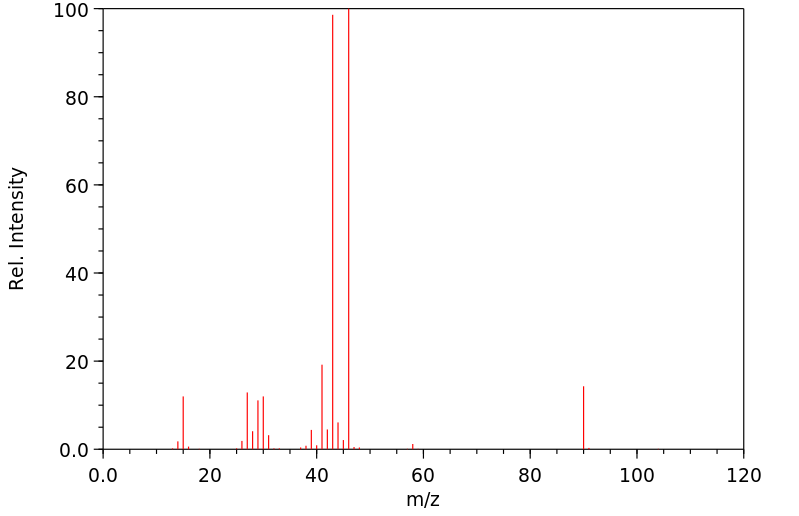
质谱MS
-
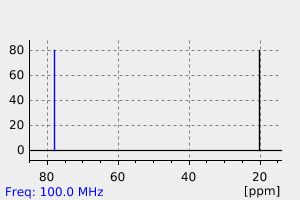
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息