硝酸异丁酯 | 543-29-3
分子结构分类
中文名称
硝酸异丁酯
中文别名
——
英文名称
isobutyl nitrate
英文别名
2-methyl-1-propyl nitrate;2-methylpropyl nitrate
CAS
543-29-3
化学式
C4H9NO3
mdl
MFCD00010444
分子量
119.12
InChiKey
LNNXFUZKZLXPOF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-111.59°C (estimate)
-
沸点:123 °C(lit.)
-
密度:1.015 g/mL at 25 °C(lit.)
-
闪点:70 °F
-
介电常数:11.9(19℃)
-
溶解度:0.01 M
-
蒸汽压力:8.30 mmHg
-
亨利常数:0.00 atm-m3/mole
-
大气OH速率常数:9.20e-13 cm3/molecule*sec
-
保留指数:739
-
稳定性/保质期:
按规格使用和储存,不会发生分解,并避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:3.1
-
危险品标志:Xn,E
-
安全说明:S15,S26,S27,S36/37/39
-
危险类别码:R5,R20/21/22,R36/37/38,R10,R1
-
WGK Germany:3
-
海关编码:2920909090
-
包装等级:II
-
危险类别:3.1
-
危险品运输编号:UN 3272 3/PG 2
-
储存条件:储存于阴凉、干燥、通风良好的库房中。远离火种和热源,避免阳光直射,并确保包装密封。应将储存物品与酸类及食用化学品分开存放,切忌混储。储区需配备适当的材料以处理可能的泄漏。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Isobutyl nitrate
CAS-No. : 543-29-3
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Flammable liquids (Category 2)
Acute toxicity, Inhalation (Category 4)
Acute toxicity, Dermal (Category 4)
Acute toxicity, Oral (Category 4)
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Explosive when dry. Heating may cause an explosion. Flammable. Harmful by inhalation, in contact with
skin and if swallowed. Irritating to eyes, respiratory system and skin.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H225 Highly flammable liquid and vapour.
H302 Harmful if swallowed.
H312 Harmful in contact with skin.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H332 Harmful if inhaled.
H335 May cause respiratory irritation.
Precautionary statement(s)
P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P280 Wear protective gloves/ protective clothing.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard information (EU)
EUH001 Explosive when dry.
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R1 Explosive when dry.
R5 Heating may cause an explosion.
R10 Flammable.
R20/21/22 Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38 Irritating to eyes, respiratory system and skin.
S-phrase(s)
S15 Keep away from heat.
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S27 Take off immediately all contaminated clothing.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C4H9NO3
Molecular Weight : 119,12 g/mol
Component Concentration
Isobutyl nitrate
CAS-No. 543-29-3 -
EC-No. 208-842-2
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13).
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, Flame retardant antistatic protective clothing, The type
of protective equipment must be selected according to the concentration and amount of the
dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: colourless
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 123 °C - lit.
boiling range
g) Flash point 21 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,015 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks. Extremes of temperature and direct sunlight.
Incompatible materials
Strong reducing agents, Strong bases
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation Harmful if inhaled. Causes respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin
Harmful if absorbed through skin. Causes skin irritation.
Eyes Causes serious eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Burn in a chemical incinerator equipped with an afterburner and scrubber but exert extra care in igniting
as this material is highly flammable. Offer surplus and non-recyclable solutions to a licensed disposal
company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3272 IMDG: 3272 IATA: 3272
UN proper shipping name
ADR/RID: ESTERS, N.O.S. (Isobutyl nitrate)
IMDG: ESTERS, N.O.S. (Isobutyl nitrate)
IATA: Esters, n.o.s. (Isobutyl nitrate)
Transport hazard class(es)
ADR/RID: 3 IMDG: 3 IATA: 3
Packaging group
ADR/RID: II IMDG: II IATA: II
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
合成制备方法
暂无相关信息。
用途暂无相关信息。
反应信息
-
作为反应物:描述:硝酸异丁酯 以3%的产率得到参考文献:名称:GANIN, EH. V.;GLINSKAYA, L. YA., YKP. XIM. ZH., 56,(1990) N, S. 323-325摘要:DOI:
-
作为产物:描述:参考文献:名称:Convenient Preparation of Alkyl Nitrates Free of Nitrites with Potassium Nitrate and Boron Trifluoride Hydrate摘要:不含亚硝酸盐的一级和二级烷基硝酸酯可通过方便地将相应醇与硝酸钾和氟硼酸钾水合物(1.25水合物)反应来制备。此方法同样成功用于制备硝酸金刚烷。DOI:10.1055/s-1993-25830
-
作为试剂:描述:S-甲基-S-苯基-N-甲基亚砜亚胺 在 硝酸异丁酯 、 对氯苯异氰酸酯 、 双(三甲基硅烷基)氨基钾 、 三乙胺 作用下, 以 四氢呋喃 、 甲苯 为溶剂, 反应 48.0h, 生成 N-methyl-S-phenylsulfinamide参考文献:名称:α-硝基砜的手性类似物α-硝基亚砜肟的制备,性质和化学反应性。摘要:外消旋的N-甲基-S-(硝基甲基)-S-苯基亚磺酰亚胺(2)是通过N,S-二甲基-S-苯基亚磺酰亚胺的碱硝化制备的,产率为87%。以类似方式制备旋光的N-甲基-S-(硝基甲基)-S-苯磺酰亚胺(两种对映体)。在呋喃存在下,外消旋物2与对氯苯基异氰酸酯和催化量的三乙胺反应,以42%的收率(65:35非对映异构体比例)得到二氢呋喃异恶唑5(丁腈氧化物环加成产物)。二氢呋喃异恶唑5与苯基锂和甲基锂的反应分别用苯基和甲基取代了亚磺酰亚胺基。外消旋物2与芳族异氰酸酯和碳酸钾的反应以70-78%的收率得到C-酰化产物,其以叶立德互变异构体9a,b的形式存在。DOI:10.1021/jo010924k
文献信息
-
Thienyl benzothienyl and dibenzothienyl compounds as inhibitors of申请人:Imperial Chemical Industries PLC公开号:US05102905A1公开(公告)日:1992-04-07The invention concerns heterocyclic nitromethane derivatives of the formula 1 as defined hereinafter (and their non-toxic salts) wherein the heterocyclic moiety Q is thienyl, benzothienyl or dibenzothienyl and may bear a wide variety of optional substituents, together with pharmaceutical compositions containing them. The nitromethane derivatives are inhibitors of the enzyme aldose reductase and are of value in the treatment of certain complications of diabetes and galactosemia.
-
Alkyl amino-glucopyranoside derivative and process for producing the same申请人:Tokyo Tanabe Company, Limited公开号:US04057684A1公开(公告)日:1977-11-08Certain alkyl amino-glucopyranoside are disclosed, including alkyl N-carbamyl-N'-(2-chloroethyl)-N'-nitroso-6-amino-6-deoxy-D-glucopyranoside , alkyl N-carbamyl-N'-(2-chloroethyl)-N'-nitroso-2-amino-2-deoxy-D-glucopyranoside , and alkyl di-N,N'-[N-(2-chloroethyl)-N-nitroso-carbamyl]-2,6-di-amino-2,6-di-deoxy-D -glucopyranoside. The alkyl amino-glucopryanoside exhibits marked antitumor activity while not having diabetogenic activity and bone marrow toxicity. The alkyl amino-glucopryanoside is further water soluble and free from antibacterial activity.
-
Process for the preparation of 4-amino- 4-3-ketosteroids via 4-nitro-申请人:Merrell Pharmaceuticals Inc.公开号:US05750744A1公开(公告)日:1998-05-12The present invention provides 4-nitro-.DELTA..sup.4 -3-ketosteroids, their use as steroid C.sub.17-20 lyase and 5.alpha.-reductase inhibitors and to a novel process for preparing a compound of the formula: ##STR1## comprising sequentially: a) reacting a starting compound of the formula ##STR2## with an effective amount of a strong base at an elevated or suitable temperature for a time sufficient to create the corresponding thermodynamic dienolate, followed by addition of a neutral nitrating agent to produce the 4-nitro-steroid; and then; b) reacting the 4-nitrosteroid with a suitable reducing agent.
-
Tricyclic heterocyclic sulfonamide and sulfonic ester derivatives申请人:Eisai Co., Ltd.公开号:US05854274A1公开(公告)日:1998-12-29Novel tricyclic heterocyclic sulfonamide derivatives and sulfonic ester derivatives which have excellent antitumor activity and are represented by the following general formula (I) and processes for producing the same are provided. The present sulfonamide derivatives or a sulfonic ester derivatives are represented by the following general formula (I): ##STR1## wherein G represents an aromatic 5- or 6-membered ring; L represents 0 or --N(R.sup.1)-- and --R.sup.1 represents hydrogen or lower alkyl; and M represents a tricyclic structure selected from among the following; ##STR2## rings A and B each represent an unsaturated 5- or 5 -membered ring; X represents N(R.sup.2) wherein R.sup.2 represents hydrogen or lower alkyl, or NHCO; Y represents 0, S(O).sub.n, C(R.sup.3) (R.sup.4), C(O), N(R.sup.5), CH(R.sup.6)CH(R.sup.7), C(R.sup.8).dbd.C(R.sup.9), N(R.sup.10) C(O), N.dbd.CR.sup.11), OCH(R.sup.12), S(O).sub.n CH(R.sup.13) or N(R.sup.14)CH(R.sup.15); Z represents nitrogen or C(R.sup.16); and n represents 0, 1 or 2, R.sup.3 to R.sub.13, R.sup.15 and R.sup.16 each represent hydrogen or lower alkyl, and R.sup.14 represents hydrogen, lower alkyl or lower acyl.本发明提供了一种具有优异抗肿瘤活性的新型三环杂环磺酰胺衍生物和磺酸酯衍生物,其由下列通式(I)所表示,并提供了制备它们的方法。所述磺酰胺衍生物或磺酸酯衍生物由以下通式(I)表示: ##STR1## 其中,G表示芳香的5-或6-成员环;L表示0或--N(R.sup.1)--,--R.sup.1表示氢或低碳基;M表示从以下所选的三环结构: ##STR2## 其中,环A和环B各自表示不饱和的5-或6-成员环;X表示N(R.sup.2),其中R.sup.2表示氢或低碳基,或者NHCO;Y表示0,S(O).sub.n,C(R.sup.3)(R.sup.4),C(O),N(R.sup.5),CH(R.sup.6)CH(R.sup.7),C(R.sup.8).dbd.C(R.sup.9),N(R.sup.10)C(O),N.dbd.CR.sup.11),OCH(R.sup.12),S(O).sub.nCH(R.sup.13)或N(R.sup.14)CH(R.sup.15);Z表示氮或C(R.sup.16);n表示0、1或2,R.sup.3至R.sub.13、R.sup.15和R.sup.16各自表示氢或低碳基,而R.sup.14表示氢、低碳基或低酰基。
-
Certain 3-ketosteroids used to inhibit steroid 5.alpha.-reductase申请人:Merrell Pharmaceuticals, Inc.公开号:US05869475A1公开(公告)日:1999-02-09The invention discloses 3-ketosteroids of the following formula used to inhibit steroid 5.alpha.-reductase: ##STR1## wherein R is OH, C.sub.1 -C.sub.6 alkanoyl, C.sub.1 -C.sub.6 alkanoyloxy, C.sub.1 -C.sub.4 alkanol, COCH.sub.2 OH, CO.sub.2 H, CONR.sub.7 R.sub.8, cyclopropoxy, acetylthioalkane, cyclopylamino, 2-2-dimethyldioxolan-4-yl, 1,2-dihydroxyethyl and C.sub.1-4 alkanethiol; R.sub.1 is hydrogen, hydroxy or C.sub.1-6 alkyl; R.sub.1 and R.sub.2 together may indicate .dbd.O, that is an oxygen double bonded to the 17 carbon; R.sub.2, R.sub.3 and R.sub.4 are each independently hydrogen or C.sub.1-6 alkyl; R.sub.5 and R.sub.6 are each independently hydrogen or OH; R.sub.5 and R.sub.6 together may indicate .dbd.O, that is an oxygen double bonded to the 11 carbon; R.sub.7 is hydrogen or C.sub.1-8 alkyl; R.sub.8 is C.sub.1-8 alkyl; and with the proviso that, when R is OH, then R.sub.1 is hydrogen; and with the proviso that, when R.sub.5 is OH, then R.sub.6 is hydrogen.该发明揭示了以下公式的3-酮类固醇,用于抑制类固醇5α-还原酶:##STR1## 其中R为OH,C.sub.1-C.sub.6烷酰基,C.sub.1-C.sub.6烷氧基,C.sub.1-C.sub.4烷醇,COCH.sub.2OH,CO.sub.2H,CONR.sub.7R.sub.8,环丙氧基,乙酰硫代烷基,环戊氨基,2-2-二甲基二氧杂环戊烷-4-基,1,2-二羟乙基和C.sub.1-4烷硫醇;R.sub.1为氢,羟基或C.sub.1-6烷基;R.sub.1和R.sub.2可以共同表示.dbd.O,即与第17碳双键的氧原子;R.sub.2,R.sub.3和R.sub.4各自独立地是氢或C.sub.1-6烷基;R.sub.5和R.sub.6各自独立地是氢或羟基;R.sub.5和R.sub.6可以共同表示.dbd.O,即与第11碳双键的氧原子;R.sub.7为氢或C.sub.1-8烷基;R.sub.8为C.sub.1-8烷基;但是,当R为OH时,R.sub.1为氢;而且,当R.sub.5为OH时,R.sub.6为氢。
表征谱图
-
氢谱1HNMR
-
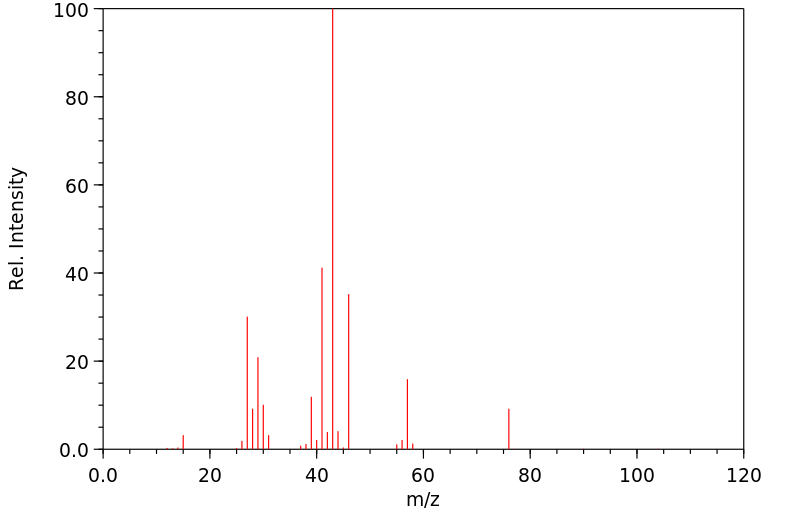
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氯酸钪
高氯酸胍
高氯酸甲酯
高氯酸四甲基铵
高氯酸四乙基铵
高氯酸吡咯烷
高氯酸三丁基锡
高氯酸[4-(2,5-二羰基吡咯烷-1-基)丁-2-炔-1-基](二甲基)锍
高氯酸2-羟乙基酯
高氯酸2-羟丙基酯
顺式-2-硝基环己基高氯酸盐
铵钪硝酸盐(2:1:5)
铵氧代-三氧代锝
铅(2+)砷酸盐-三丁基锡烷基水合物(3:2:2:1)
钪三溴酸盐
钠O-(四氢-2-呋喃基甲基)硫代硫酸酯
重铬酸咪唑
重铬酸吡啶
过氧硝酸甲酯
过氧硝酸环戊酯
过氧化,硝基1-羰基-2-丙烯基
过氧化,甲磺酰硝基
过氧丙酰硝酸酯
膦酸二异丁基酯
磷酸组胺
磷酸(羟基-甲氧基-磷酰)二甲酯
磷酸(羟基-异戊氧基磷酰)二异戊基酯
碳化钨
碲烷
碲杂环丁烷
碲吩-2-羧酸
碲吩
硼酸异辛酯
硼酸十六烷基酯
硼酸三辛酯
硼酸三甲酯
硼酸三烯丙酯
硼酸三正己酯
硼酸三月桂酯
硼酸三异丙酯
硼酸三庚酯
硼酸三亚甲酯
硼酸三乙酯
硼酸三丙酯
硼酸三丁酯-11B
硼酸三丁酯-10B
硼酸三丁酯
硼酸三-[3-(3-甲氧基-丙氧基)-丙基]酯
硼酸三-(2-氯甲氧基-乙基)酯
硼酸三(六氟异丙基)酯