5,6-二氯-2-甲基苯并咪唑 | 6478-79-1
中文名称
5,6-二氯-2-甲基苯并咪唑
中文别名
2-甲基-5,6-二氯苯并咪唑
英文名称
5,6-dichloro-2-methylbenzimidazole
英文别名
5,6-dichloro-2-methyl-1H-benzimidazole;5,6-dichloro-2-methyl-benzoimidazole;5,6-dichloro-2-methyl-1H-benzo[d]imidazole;2-methyl-5,6-dichlorobenzimidazole
CAS
6478-79-1
化学式
C8H6Cl2N2
mdl
——
分子量
201.055
InChiKey
WMOBNOCVMZFPEN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:238-240
-
沸点:409.0±25.0 °C(Predicted)
-
密度:1.486
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:28.7
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温条件下。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 5,6-Dichloro-2-methylbenzoimidazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5,6-Dichloro-2-methylbenzoimidazole
CAS number: 6478-79-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H6Cl2N2
Molecular weight: 201.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 5,6-Dichloro-2-methylbenzoimidazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5,6-Dichloro-2-methylbenzoimidazole
CAS number: 6478-79-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H6Cl2N2
Molecular weight: 201.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5,6-dichloro-2-[2-(tetrahydrofuran-2-yl)-ethyl]-1H-benzoimidazole 906639-94-9 C13H14Cl2N2O 285.173 5,6-二氯-1,2-二甲基苯并咪唑 5,6-dichloro-1,2-dimethyl-1H-benzimidazole 2818-64-6 C9H8Cl2N2 215.082 —— 5,6-dichloro-2-(2-(furan-2-yl)-ethyl)-1H-benzoimidazole 906639-93-8 C13H10Cl2N2O 281.141 —— 5,6-dichloro-2-(2-(thiophen-2-yl)-ethyl)-1H-benzoimidazole 906639-95-0 C13H10Cl2N2S 297.208 —— 5,6-dichloro-4,7-diiodo-2-methyl-1H-benzimidazole 1196457-14-3 C8H4Cl2I2N2 452.848 —— 2-(5,6-dichloro-1H-benzoimidazol-2-yl)-1-(furan-2-yl)-ethanol 906639-70-1 C13H10Cl2N2O2 297.141 —— 2-(5,6-dichloro-1H-benzoimidazol-2-yl)-1-(thiophen-2-yl)-ethanol 906639-71-2 C13H10Cl2N2OS 313.207
反应信息
-
作为反应物:描述:5,6-二氯-2-甲基苯并咪唑 在 水 、 氢溴酸 、 potassium hydroxide 作用下, 以 二氯甲烷 、 丙酮 为溶剂, 反应 26.0h, 生成 1-(5-carboxypentyl)-5,6-dichloro-2,3-dimethyl-benzoimidazolium bromide参考文献:名称:Benzimidazolium dyes and their use as fluorescent chemosensors摘要:本发明涉及以下式(I)的苯并咪唑盐染料化合物:其中,n为2-10的整数,m为2-10的整数,X1和X2分别为卤素,Q为H或树脂,R为(aromatic)o-(linker)p-,其中linker为饱和或不饱和的C1-C5烃链,每个芳香基独立地为取代或未取代的芳香或杂芳基,o为1或2,p为0或1。还公开了制备和使用这些化合物的方法。公开号:US08716491B2
-
作为产物:描述:参考文献:名称:盐酸羟胺活化邻氨基苯甲酰胺/苯二胺和DMF衍生物一锅法合成1,3-二氮杂环摘要:有机合成化学中非常需要开发可持续方法。在这方面,我们报道了盐酸羟胺介导的1,3-二氮杂环化合物的一锅法合成,使用邻氨基苯甲酰胺或邻苯二胺,在无溶剂条件下于120-140℃下进行酰胺转酰胺基化8-14小时。该方法在无金属和氧化剂的条件下顺利进行,表现出广泛的底物范围,涉及二胺衍生物和酰胺,以良好至优异的产率产生相应的 4(3)-喹唑啉酮(和苯并咪唑 (&)(~47 个例子)) (56–96 %)。作为一种高效且环保的方式获得多种 4(3)-喹唑啉酮和苯并咪唑,该方法的合成实用性已通过克级操作得到证明,收率 >90 %。DOI:10.1016/j.tet.2024.133866
文献信息
-
Rhodium catalyzed 2‐alkyl‐benzimidazoles synthesis from benzene‐1,2‐diamines and tertiary alkylamines as alkylating agents作者:Yamini、Saurabh Sharma、Pralay DasDOI:10.1002/aoc.6278日期:2021.8synthesized from benzene-1,2-diamine and tertiary amines as alkylating agent under polystyrene supported rhodium (Rh@PS) nanoparticles (NPs) catalyzed conditions. The heterogeneous rhodium catalyst was applied first time for the synthesis of 2-alkyl-benzimidazoles. The reaction followed through oxidation of alkylamines, transamination, and oxidative cyclisation with benzene-1,2-diamines for the corresponding
-
Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives作者:Zongjie Gan、Qingqiang Tian、Suqin Shang、Wen Luo、Zeshu Dai、Huajun Wang、Dan Li、Xuetong Wang、Jianyong YuanDOI:10.1016/j.tet.2018.11.014日期:2018.12general, and economical synthesis of diversely functionalized benzimidazoles and 2-substituted benzimidazoles has been realized via the imidazolium chloride-catalyzed cyclization of o-phenylenediamines with DMF derivatives. This protocol shows a broad substrate scope for aliphatic, aromatic, and heteroaromatic amides. A series of benzimidazoles and 2-substituted benzimidazoles have been obtained in moderate
-
一种苯并咪唑及其衍生物的合成方法
-
Synthesis and Anti-Hepatitis B Virus Activity of Novel Benzimidazole Derivatives作者:Yun-Fei Li、Gui-Feng Wang、Pei-Lan He、Wei-Gang Huang、Feng-Hua Zhu、He-Yong Gao、Wei Tang、Yu Luo、Chun-Lan Feng、Li-Ping Shi、Yu-Dan Ren、Wei Lu、Jian-Ping ZuoDOI:10.1021/jm060330f日期:2006.7.1A series of novel benzimidazole derivatives was synthesized and evaluated for their anti-hepatitis B virus (HBV) activity and cytotoxicity in vitro. Strong activity against HBV replication and low cytotoxicity were generally observed in these benzimidazoles. The most promising compounds were 12a and 12b, with similar high antiviral potency (IC50 = 0.9 and 0.7 microM, respectively) and remarkable selectivity
-
Simple inorganic base promoted C–N and C–C formation: synthesis of benzo[4,5]imidazo[1,2-<i>a</i>]pyridines as functional AIEgens used for detecting picric acid作者:Kai Yang、Shi-He Luo、Si-Hong Chen、Xi-Ying Cao、Yong-Jun Zhou、Yan-Lan Lin、Yan-Ping Huo、Zhao-Yang WangDOI:10.1039/d1ob01424b日期:——Metal-free catalyzed intermolecular tandem Michael addition/cyclization has been developed for the synthesis of benzo[4,5]imidazo[1,2-a]pyridines from α-bromocinnamaldehyde and 2-substituted benzimidazoles. The reaction promoted by a simple inorganic base displays moderate to good yields and good functional group tolerance. The optical properties of some typical products have been investigated. We无金属催化分子间串联迈克尔加成/环化已被开发用于从 α-溴肉桂醛和 2-取代苯并咪唑合成苯并[4,5]咪唑并[1,2- a ]吡啶。由简单的无机碱促进的反应显示出中等至良好的产率和良好的官能团耐受性。研究了一些典型产品的光学特性。我们发现,由于苯并[4,5]咪唑并[1,2- a ]吡啶的C1位存在限制分子内运动的苯环,作为一种新型的聚集诱导发射(AIE) luminogen (AIEgen),它们显示出非常好的固态荧光,量子产率高达 88.80%。重要的是,化合物3b的 AIE 性能可用于检测硝基芳香炸药苦味酸 (PA),检测限和淬灭常数分别为 42.5 nM 和 7.27 × 10 4 M -M。
表征谱图
-
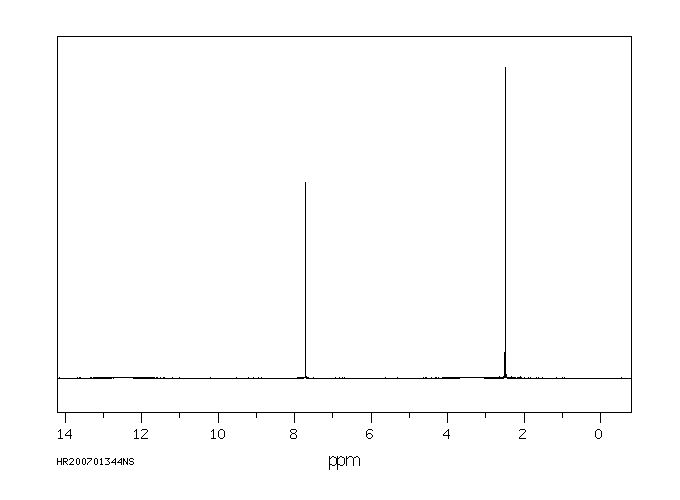
氢谱1HNMR
-
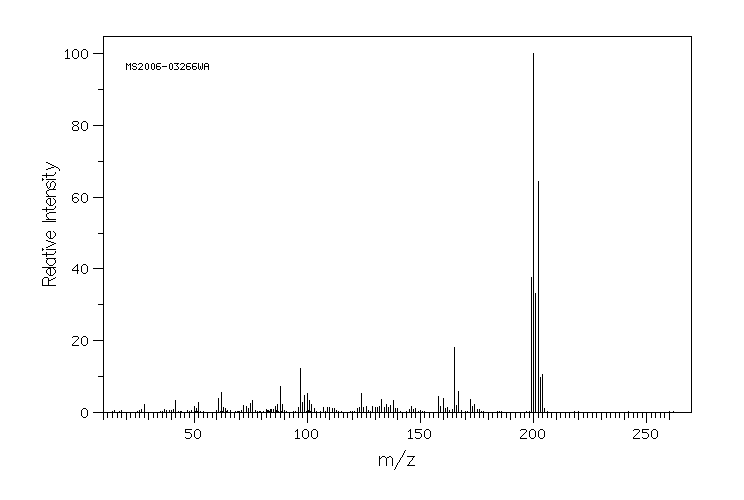
质谱MS
-
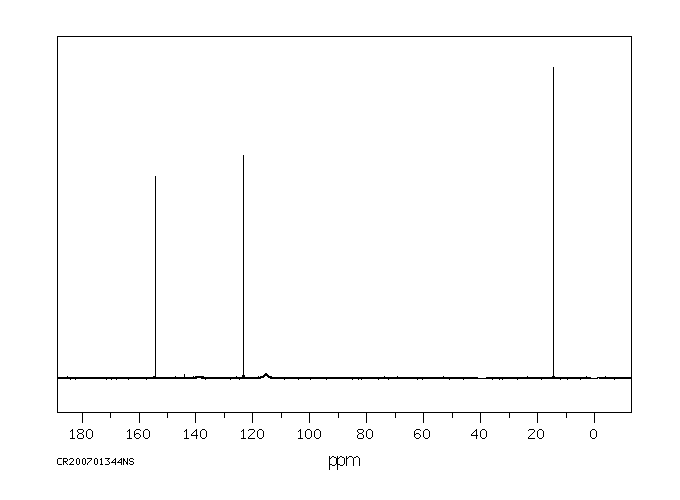
碳谱13CNMR
-
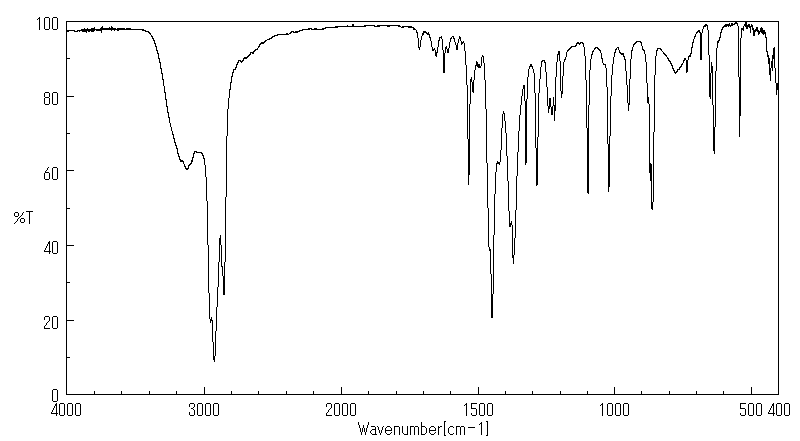
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10