2-羟基-2-环戊烯-1-酮 | 10493-98-8
中文名称
2-羟基-2-环戊烯-1-酮
中文别名
——
英文名称
2-hydroxy-2-cyclopenten-1-one
英文别名
2-hydroxycyclopent-2-en-1-one;2-hydroxycyclopent-2-enone;2-cyclopenten-1-one, 2-hydroxy-;4RS-hydroxy-cyclopent-2-enone
CAS
10493-98-8
化学式
C5H6O2
mdl
——
分子量
98.1014
InChiKey
WOPKYMRPOKFYNI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52-53℃
-
沸点:245℃
-
密度:1.334
-
闪点:101℃
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:-0.290 (est)
-
保留指数:919;905
-
稳定性/保质期:
存在于烤烟烟叶中。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2914400090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:2-8°C
SDS
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基环戊-2-烯-1-酮 2-methoxycyclopent-2-en-1-one 22323-97-3 C6H8O2 112.128
反应信息
-
作为反应物:描述:2-羟基-2-环戊烯-1-酮 以94%的产率得到3-甲基-2-环戊烯-1-酮参考文献:名称:Process for preparing oxocyclopentene derivatives摘要:制备氧代环戊烯的方法,其化学式为:其中R.sup.1为氢、低烷基或低烯基,R.sup.2为氢、低烷基、低烯基、低炔基、取代或未取代芳基、芳基(低)烷基、噻吩基或环烷基,包括将化学式为的呋喃-碳醇经过重排,得到化学式为的羟基环戊烯酮,然后将化学式为的羟基环戊烷酮进行氢化,最后将化学式为的羟基环戊酮进行脱水。公开号:US04970345A1
-
作为产物:描述:cis-1,2-cyclopentanediol 在 lithium hydroxide monohydrate 、 1% platinum on charcoal 、 水 作用下, 以 乙腈 为溶剂, 反应 4.0h, 以19%的产率得到2-羟基-2-环戊烯-1-酮参考文献:名称:Heterogeneous platinum catalytic aerobic oxidation of cyclopentane-1,2-diols to cyclopentane-1,2-diones摘要:A method for the aerobic oxidation of cyclopentane-1,2-diols to the corresponding diketones over a commercial heterogeneous Pt/C catalyst is described. Unsubstituted and 3- or 4-substituted cyclopentane-1,2-diols are oxidized to 1,2-dicarbonyl compounds in good yields under the reported optimized reaction conditions (atmospheric air, 1 mol % of catalyst, 1 equiv of LiOH, aqueous solvents and 60 degrees C temperature). The method is applicable for producing cyclopentane-1,2-diketones in a scalable manner. (C) 2014 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2014.03.104
文献信息
-
Synthetic and Theoretical Studies of Cyclobuta[1,2:3,4]dicyclopentene. Organocobalt Intermediates in the Construction of the Unsaturated Carbon Skeleton and Their Transformation into Novel Cobaltacyclic Complexes by C−C Insertion作者:Andrew G. Myers、Miki Sogi、Michael A. Lewis、Stephen P. ArvedsonDOI:10.1021/jo030368p日期:2004.4.1kcal/mol higher in energy than the isomeric hydrocarbon 1,6-didehydro[10]annulene (2), a molecule known to isomerize to 1,5-didehydronaphthalene (4) above −50 °C. Calculated enthalpic changes of homodesmotic reactions support the notion that 1 is an aromatic molecule with a resonance stabilization energy (RSE) about half to two-thirds that of benzene on a per-molecule basis. Investigations of potential描述了三环10π-电子烃环丁[1,2:3,4]二环戊烯(1)的理论和合成研究,这是一种从未合成的名义上的芳香结构。通过密度泛函理论计算(B3LYP / 6-31G(d,p))进行的几何优化预测1是具有非交替的C单键和双键的D 2 h对称结构。计算还预测,1为4.7千卡/摩尔的能量比异构烃1,6-二脱氢更高[10]轮烯(2),公知的异构化为1,5- didehydronaphthalene(分子4高于-50)℃的。计算的同态反应的焓变支持以下观点:1是在每个分子的基础上具有约苯的共振稳定能(RSE)的芳香族分子的三分之二至三分之二。对1的潜在合成途径的研究最初将三环碳酸酯11(一种分子内[2 + 2]-光环化反应的产物)用作原料。在这些研究中,将11分几步转化为二萘烷12,该二萘烷经氟化硼乙醚醚处理后形成了不稳定的烃双环戊二烯基(13)。为了避免不饱和三环的中心四元环的断裂[5.3.0.0 2
-
Asymmetric Organocatalytic Michael Addition–Cyclization Cascade of Cyclopentane-1,2-dione with Substituted α,β-Unsaturated Aldehydes作者:Margus Lopp、Gert Preegel、Estelle Silm、Sandra Kaabel、Ivar Järving、Kari RissanenDOI:10.1055/s-0036-1588787日期:2017.7yields and excellent enantioselectivity. An asymmetric organocatalytic Michael addition–cyclization cascade reaction has been developed using cyclopentane-1,2-dione as a Michael donor and α,β-unsaturated aldehydes as Michael acceptors. Bicyclic hemiacetals were obtained in excellent yields and enantioselectivities. On the basis of the results, a one-pot reaction has been developed to obtain chiral 3-substituted
-
Enantioselective Organocatalytic Michael Addition of Cyclopentane-1,2-diones to Nitroolefins作者:Margus Lopp、Gert Preegel、Artur Noole、Kaja Ilmarinen、Ivar Järving、Tõnis Kanger、Tõnis PehkDOI:10.1055/s-0034-1378374日期:——Abstract Organocatalytic Michael additions of cyclopentane-1,2-dione to different nitroolefins have been investigated. Cyclopentane-1,2-dione undergoes an organocatalytic reaction with substituted nitroolefins giving 3-substituted products in good to high yields (48–97%) and good stereoselectivity (up to 76% ee). Organocatalytic Michael additions of cyclopentane-1,2-dione to different nitroolefins have
-
Anhydrous Iron(III) Chloride Dispersed on Silica Gel; III.<sup>1,2</sup>A Convenient and Mild Reagent for Deacetalization in Dry Medium
-
Asymmetric Synthesis of Chiral 1,3-Diaminopropanols: Bisoxazolidine-Catalyzed CC Bond Formation with α-Keto Amides作者:Hanhui Xu、Christian WolfDOI:10.1002/anie.201105778日期:2011.12.16Three high‐yielding steps lead to the formation of chiral 1,3‐diaminopropanols from aliphatic and aromatic α‐keto amides. In this approach, a nitroaldol reaction, which is catalyzed by Cu(SO2CF3)2 and the bisoxazolidine ligand L1, is followed by two mild reduction reactions (see scheme). Laborious protection and deprotection steps can be avoided by using this method.
表征谱图
-
氢谱1HNMR
-
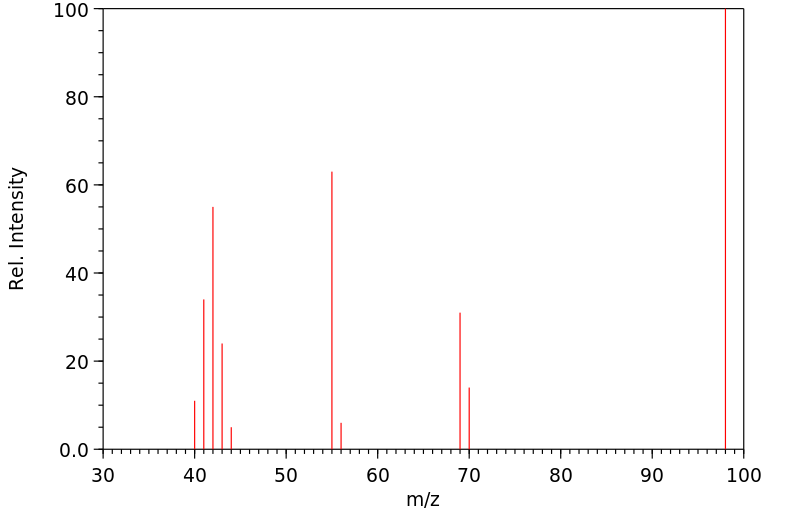
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷