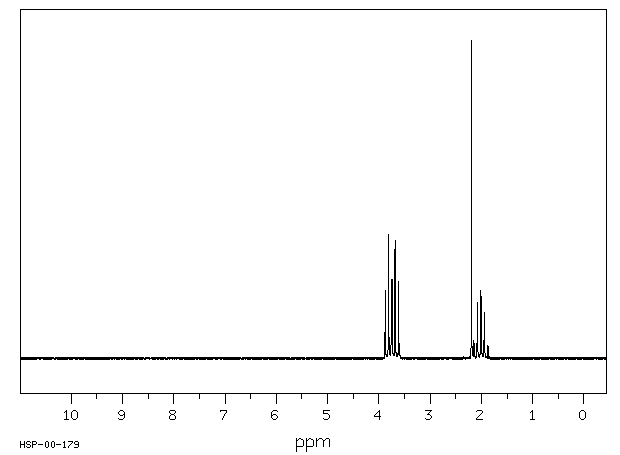
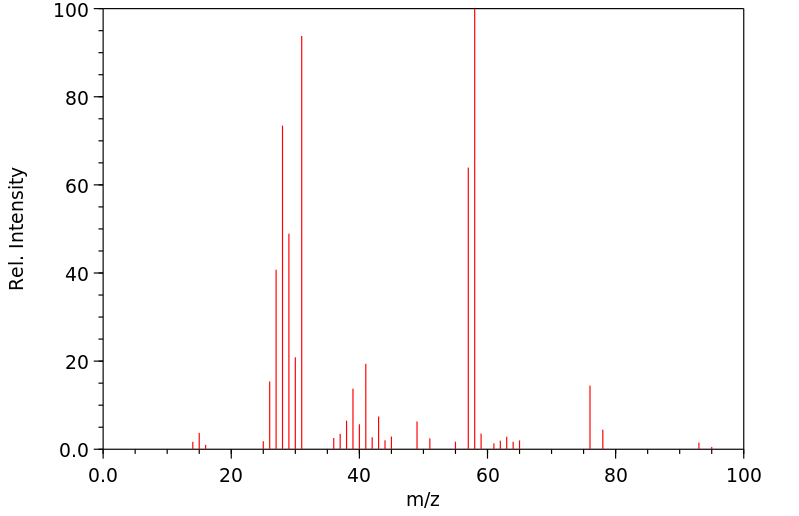
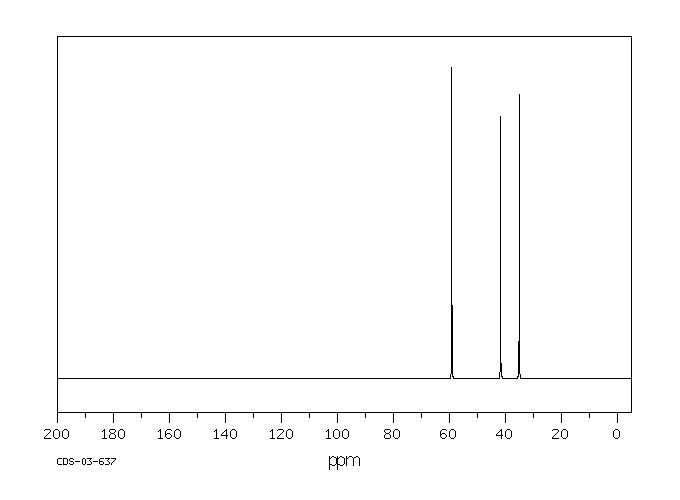
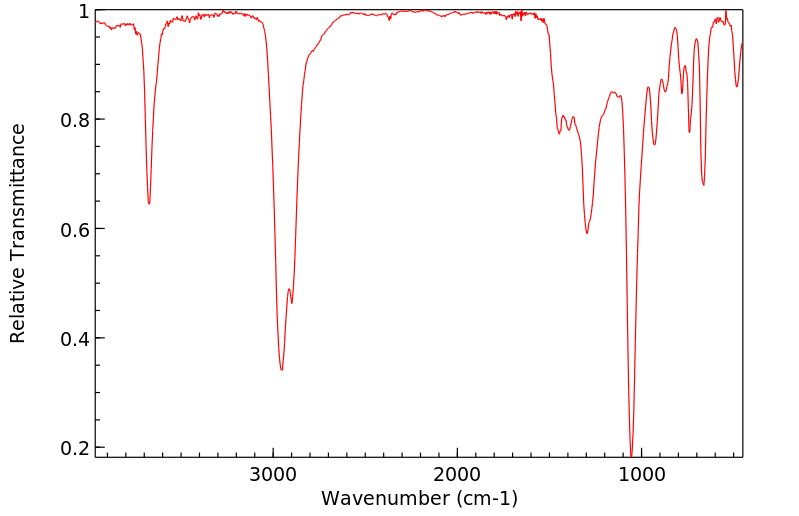
equation log (k/ko)=ρq · σ is applied to the solvolysis of the 3-substituted propyl bromides 6a-6i in ethanol/water 4:1 (v/v) log k correlates linearly with σ except in cases where R exerts an anchimeric effect. The reaction constant ρq for 6 is − 0.12 and is typical for a nucleophilic solvent-assisted ks process at a primary C-atom. The tertiary halides 1 and 3, however, which react with little or no nucleophilic
当哈米特-塔夫脱方程日志(K /ķ ö)=ρ q ·σ被施加到3-取代的丙基
溴化物的溶剂分解图6a-6i的在
乙醇/
水4:1 (V / V)登录ķ相关因素线性σ除外,其中R发挥
邻氨基苯甲酸作用。反应常数ρ q为6为- 0.12,是典型的亲核溶剂辅助ķ小号过程在一个主C-原子。但是,叔卤化物1和3与亲核溶剂的辅助作用很少或没有亲核反应,即通过k c过程,导致较大的ρ q分别-0.71和-1.14,值。因此,反应常数p q是在饱和状态下对饱和化合物进行溶剂化的电荷发展的灵敏指标。