5-氟-2-羟基苯乙酮 | 394-32-1
中文名称
5-氟-2-羟基苯乙酮
中文别名
2-乙酰基-4-氟苯酚;2'-羟基-5'-氟苯乙酮;5'-氟-2'-羟基苯乙酮;2-羟基-5-氟苯乙酮
英文名称
1-(5-fluoro-2-hydroxyphenyl)ethan-1-one
英文别名
5-fluoro-2-hydroxyacetophenone;1-(5-fluoro-2-hydroxyphenyl)ethanone;1–(5-fluoro-2-hydroxyphenyl)ethan-1-one;5'-Fluoro-2'-hydroxyacetophenone
CAS
394-32-1
化学式
C8H7FO2
mdl
MFCD00011668
分子量
154.141
InChiKey
KOFFXZYMDLWRHX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56-58 °C(lit.)
-
沸点:65-66 °C8 mm Hg(lit.)
-
密度:1.1850 (estimate)
-
闪点:65-66°C/8mm
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:AM8502300
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:| 室温 |
SDS
| Name: | 5 -Fluoro-2 -Hydroxyacetophenone 97% Material Safety Data Sheet |
| Synonym: | 1-(5-Fluoro-2-Hydroxyphenyl)-1-Ethanone |
| CAS: | 394-32-1 |
Synonym:1-(5-Fluoro-2-Hydroxyphenyl)-1-Ethanone
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 394-32-1 | 5'-Fluoro-2'-Hydroxyacetophenone | 97 | 206-893-5 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 394-32-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: yellow to brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 65.0 - 66.0 deg C @ 8.00mmHg
Freezing/Melting Point: 53.00 - 56.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H7FO2
Molecular Weight: 154.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 394-32-1: AM8502300 LD50/LC50:
CAS# 394-32-1: Oral, mouse: LD50 = >1 gm/kg.
Carcinogenicity:
5'-Fluoro-2'-Hydroxyacetophenone - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 394-32-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 394-32-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 394-32-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5’-氟-2’-甲氧基苯乙酮 1-(5-fluoro-2-methoxyphenyl)ethanone 445-82-9 C9H9FO2 168.168 2'-羟基苯乙酮 o-hydroxyacetophenone 118-93-4 C8H8O2 136.15 —— 1-(2-(benzyloxy)-5-fluorophenyl)ethan-1-one 1799-18-4 C15H13FO2 244.265 2-羟基-5-氯苯乙酮 5-chloro-2-hydroxyacetophenone 1450-74-4 C8H7ClO2 170.595 2-羟基-5-溴苯乙酮 5-Bromo-2-hydroxyacetophenone 1450-75-5 C8H7BrO2 215.046 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-5′-氟-2′-羟基苯乙酮 α-bromo-(2-hydroxy-5-fluoro)acetophenone 126581-65-5 C8H6BrFO2 233.037 5’-氟-2’-甲氧基苯乙酮 1-(5-fluoro-2-methoxyphenyl)ethanone 445-82-9 C9H9FO2 168.168 —— 3-(5-fluoro-2-hydroxyphenyl)-3-oxopropanal —— C9H7FO3 182.151 1-(3-乙酰基-5-氟-2-羟基苯基)乙酮 1-(3-acetyl-5-fluoro-2-hydroxyphenyl)ethanone 106823-62-5 C10H9FO3 196.178 —— 1-(5-Fluoro-2-hydroxyphenyl)-3-phenylpropan-1-one 1341230-18-9 C15H13FO2 244.265 —— 3-(dimethylamino)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one —— C11H12FNO2 209.22 —— 2-Aethoxy-5-fluor-acetophenon 1466-79-1 C10H11FO2 182.195 3-二甲氨基-1-(5-氟-2-羟基苯基)丙烯酮 (E)-3-(dimethylamino)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one 904318-52-1 C11H12FNO2 209.22 2-乙基-4-氟苯酚 2-ethyl-4-fluorophenol 398-71-0 C8H9FO 140.157 —— 1-(5-Fluoro-2-hydroxyphenyl)-3-(4-fluorophenyl)propan-1-one 1341230-19-0 C15H12F2O2 262.256 —— 1-(2-(difluoromethoxy)-5-fluorophenyl)ethan-1-one 1214338-07-4 C9H7F3O2 204.149 —— 1-(5-fluoro-2-hydroxyphenyl)butane-1,3-dione —— C10H9FO3 196.178 1-(5-氟-2-羟基苯基)-3-苯基-1,3-丙烷二酮 1-(5-fluoro-2-hydroxyphenyl)-3-phenylpropane-1,3-dione 58483-26-4 C15H11FO3 258.249 —— 5-Fluor-2-<2-hydroxy-ethoxy>-1-acetyl-benzol 776-83-0 C10H11FO3 198.194 —— 1-[2-(allyloxy)-5-fluorophenyl]ethanone 1092306-36-9 C11H11FO2 194.206 —— 1-(2-(2-bromoethoxy)-5-fluorophenyl)ethan-1-one 1282530-48-6 C10H10BrFO2 261.091 —— 2-chloro-1-(5-fluoro-2-methoxyphenyl)ethanone 854036-06-9 C9H8ClFO2 202.613 —— 1-(3-chloro-5-fluoro-2-hydroxyphenyl)ethanone 445-38-5 C8H6ClFO2 188.586 —— 5'-Fluor-2'-hydroxy-chalkon 1431-92-1 C15H11FO2 242.25 —— (E)-1-(2-hydroxy-5-fluorophenyl)-3-phenylprop-2-en-1-one 1431-92-1 C15H11FO2 242.25 —— 1-[2-(but-3-yn-1-yloxy)-5-fluorophenyl]ethanone 1540416-96-3 C12H11FO2 206.217 —— 1-(5-fluoro-2-hydroxyphenyl)-3-(4-fluorophenyl)propane-1,3-dione 1225785-23-8 C15H10F2O3 276.239 —— 1-(3-Amino-5-fluoro-2-hydroxyphenyl)ethan-1-one 70977-84-3 C8H8FNO2 169.155 —— 1-(3-bromo-5-fluoro-2-hydroxyphenyl)ethanone 393-62-4 C8H6BrFO2 233.037 —— (E)-1-(2-hydroxy-5-fluorophenyl)-3-(4-fluorophenyl)prop-2-en-1-one 224294-26-2 C15H10F2O2 260.24 —— 1-(5-Fluoro-2-hydroxyphenyl)-3-(4-fluorophenyl)prop-2-en-1-one 93513-88-3 C15H10F2O2 260.24 —— 1-[2-(pent-4-yn-1-yloxy)-5-fluorophenyl]ethanone 1454850-00-0 C13H13FO2 220.243 —— (E,E)-5'-fluoro-2'-hydroxycinnamylideneacetophenone 386212-35-7 C17H13FO2 268.287 —— acetic acid 2-acetyl-4-fluorophenyl ester 106823-61-4 C10H9FO3 196.178 —— 1-(2-(benzyloxy)-5-fluorophenyl)ethan-1-one 1799-18-4 C15H13FO2 244.265 2-(2-乙酰基-4-氟苯氧基)乙酸 2-(2-acetyl-4-fluorophenoxy)acetic acid 34848-65-2 C10H9FO4 212.177 —— 1-(5-fluoro-2-phenethoxyphenyl)ethanone 1039969-15-7 C16H15FO2 258.292 —— 5’-fluoro-2’,2-dihydroxychalcone 607-84-1 C15H11FO3 258.249 3-(4-氯苯基)-1-(5-氟-2-羟基苯基)丙-2-烯-1-酮 (E)-3-(4-chlorophenyl)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one 850799-78-9 C15H10ClFO2 276.695 —— α-morpholino-(2-hydroxy-5-fluoro)acetophenone 126581-64-4 C12H14FNO3 239.246 —— 1-(5-Fluor-2-hydroxy-phenyl)-3-(4-methoxy-phenyl)-prop-2-enon 391-94-6 C16H13FO3 272.276 —— (E)-1-(2-hydroxy-5-fluorophenyl)-3-(4-methoxyphenyl)prop-2-en-1-one 391-94-6 C16H13FO3 272.276 —— 3-(4-Bromophenyl)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one 93513-90-7 C15H10BrFO2 321.146 —— 5-fluoro-2-(oxiranylmethoxy)acetophenone 57339-49-8 C11H11FO3 210.205 —— 1-(5-fluoro-2-((R)-2-oxiranylmethoxy)phenyl)ethanone 1106940-62-8 C11H11FO3 210.205 —— 3-(2-fluorophenyl)-1-(5-fluoro-2-hydroxyphenyl)-2-propen-1-one 224294-29-5 C15H10F2O2 260.24 —— (2E,4E)-1-(5-fluoro-2-hydroxyphenyl)-5-(4-methoxyphenyl)penta-2,4-dien-1-one 1422364-10-0 C18H15FO3 298.314 2-(2-乙酰基-4-氟苯氧基)乙酸乙酯 (2-acetyl-4-fluorophenoxy)acetic acid ethyl ester 34849-57-5 C12H13FO4 240.231 —— (E)-1-(2-hydroxy-5-fluorophenyl)-3-(3-methoxyphenyl)prop-2-en-1-one 1381931-08-3 C16H13FO3 272.276 6-氟-4-二氢色原酮 6-fluoro-4-chromanone 66892-34-0 C9H7FO2 166.152 —— 4-Fluoro-2-((R)-1-hydroxy-ethyl)-phenol 156597-63-6 C8H9FO2 156.157 1-(2-羟基-5-氟苯基)乙醇 4-fluoro-2-(1-hydroxyethyl)phenol 850793-83-8 C8H9FO2 156.157 —— (E)-1-(5-Fluoro-2-hydroxy-phenyl)-3-furan-2-yl-propenone 43191-65-7 C13H9FO3 232.211 —— 1-[3-acetyl-2-(2-chloroethoxy)-5-fluorophenyl]ethanone 737002-24-3 C12H12ClFO3 258.677 —— 5'-fluoro-2'-hydroxy-2-methoxychalcone —— C16H13FO3 272.276 —— 2-acetyl-4-flurophenyl dimethylcarbamate 1416426-09-9 C11H12FNO3 225.22 —— (E)-1-(5-Fluoro-2-hydroxy-phenyl)-3-pyridin-3-yl-propenone —— C14H10FNO2 243.237 —— 1-<5-Fluor-2-hydroxy-phenyl>-3-<4-dimethylamino-phenyl>-prop-2-en-on 2559-04-8 C17H16FNO2 285.318 —— (2Z,4E)-1-(5-fluoro-2-hydroxyphenyl)-3-hydroxy-5-phenylpenta-2,4-dien-1-one 869563-65-5 C17H13FO3 284.287 5-氟水杨酸 5-fluoro-2-hydroxybenzoic acid 345-16-4 C7H5FO3 156.113 7-氟-3,4-二氢-2H-苯并[b]氧杂环庚三烯-5-酮 7-fluoro-3,4-dihydro-2H-benzo[b]oxepin-5-one 774-20-9 C10H9FO2 180.179 —— 1-(5-fluoro-2-hydroxyphenyl)-3-(pyridine-3-yl)propane-1,3-dione 1443119-34-3 C14H10FNO3 259.237 —— 1-(5-Fluor-2-hydroxy-phenyl)-3-(4-hydroxy-3-methoxy-phenyl)-prop-2-enon 847-13-2 C16H13FO4 288.275 —— 5-fluoro-2-hydroxybenzofuran-3(2H)-one 1613044-62-4 C8H5FO3 168.124 —— 5'-fluoro-2'-hydroxy-2,4-dimethoxychalcone 577764-86-4 C17H15FO4 302.302 1-(5-氟-2-甲氧基苯基)乙醇 1-(5-fluoro-2-methoxyphenyl)-1-ethanol 878572-08-8 C9H11FO2 170.184 —— 2-(2-ethyl-4-fluorophenoxy)acetic acid 582-12-7 C10H11FO3 198.194 —— 2-acetyl-4-fluorophenyl triflate 874388-48-4 C9H6F4O4S 286.204 —— 1-[5-Fluoro-2-(oxiran-2-ylmethoxy)phenyl]-3-phenylpropan-1-one 1341230-20-3 C18H17FO3 300.33 6-氟-2-甲基-4-苯并二氢吡喃-4-酮 6-fluoro-2-methyl-4-chromanone 88754-96-5 C10H9FO2 180.179 —— (E)-1-(5-fluoro-2-hydroxyphenyl)-3-(3-nitrophenyl)prop-2-en-1-one 1215089-80-7 C15H10FNO4 287.247 —— 1-[5-Fluoro-2-(oxiran-2-ylmethoxy)phenyl]-3-(4-fluorophenyl)propan-1-one 1341230-22-5 C18H16F2O3 318.32 —— 5'-fluoro-2'-hydroxy-3,4,5-trimethoxy-chalcone 527751-44-6 C18H17FO5 332.328 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:5-氟-2-羟基苯乙酮 在 (S,Sp)-RuPHOX-Ru 、 氢气 、 sodium hydride 、 sodium carbonate 作用下, 以 四氢呋喃 、 甲醇 、 mineral oil 为溶剂, 20.0 ℃ 、2.0 MPa 条件下, 反应 26.08h, 生成 4-chromanol参考文献:名称:通过RuPHOX-Ru催化色酮的不对称氢化 合成手性色酚†摘要:通过RuPHOX-Ru催化色酮的不对称氢化,以高产率,> 20:1 drs和最高99.9%ee合成了手性苯甲酚及其衍生物。对照实验表明,该反应经历了C C和C O双键的两个连续的不对称氢化步骤。该反应可以以克级进行,具有相对较低的催化剂负载量(最高1000 S / C),并且所得产物可以转化为几种生物活性化合物。DOI:10.1039/c8cc07787h
-
作为产物:参考文献:名称:2,5-二取代的1,3,4-恶二唑衍生物的合成及其体外抗炎,抗氧化作用及分子对接研究。摘要:通过环化合成了一系列新颖的2、5-二取代的1、3、4-Oxadiazole衍生物作为潜在的抗炎药和抗氧化剂。采用传统方法,在三氯氧磷(POCl3)存在下,用取代酸处理过的酰肼分子是一种有效的试剂和溶剂,反应时间短,产率高。与标准药物相比,新合成的1、3、4-恶二唑衍生物表现出优异的抗炎和抗氧化活性。对关键的抗炎靶标环氧合酶2(COX-2)的分子对接研究表明,支架能够正确识别活性位点,并与其中的关键残基实现显着的键合和非键合相互作用。DOI:10.1016/j.bmcl.2020.127136
-
作为试剂:描述:2-三氟甲基苯甲酰氯 在 氢氧化钾 、 5-氟-2-羟基苯乙酮 、 一水合肼 作用下, 以 吡啶 、 乙醇 为溶剂, 反应 27.0h, 以30%的产率得到4-fluoro-2-[5(2-trifluoromethylphenyl)-1H-pyrazol-3-yl]phenol参考文献:名称:[EN] PIRAZOLE MODULATORS OF ATP-BINDING CASSETTE TRANSPORTERS
[FR] PYRAZOLES, MODULATEURS DE TRANSPORTEURS DE CASSETTES DE LIAISON A L'ATP摘要:本发明涉及式(I)的吡唑衍生物,它们是ATP结合盒('ABC')转运蛋白或其片段的调节剂,包括囊性纤维化跨膜调节蛋白('CFTR'),以及相关的组合物和方法。本发明还涉及使用这些调节剂治疗ABC转运蛋白介导的疾病的方法。公开号:WO2004080972A1
文献信息
-
[EN] PROCESSES FOR THE PREPARATION OF FUNGICIDAL COMPOUNDS<br/>[FR] PROCÉDÉS DE PRÉPARATION DE COMPOSÉS FONGICIDES申请人:GILEAD APOLLO LLC公开号:WO2018161008A1公开(公告)日:2018-09-07Provided herein are processes for the preparation of stereomerically enriched compounds of Formulas I-014, I-020, I-064, I-074, I-082, I-089, I-090, I-095, I-171, I-181, I-184, I-186, I-189, I-191, I-192, I-193, I-205, I-206, I-208, I-211, I-212, I-213, I-220, I-229, I-231, I-233, I-234, I-246, I-251, I-258, I-259, I-262, I-263, I-285, I-323 and I-400. The compounds described herein exhibit activity as pesticides and are useful, for example, in methods for the control of fungal pathogens and diseases caused by fungal pathogens in plants. A preferred process is directed to preparing a stereomerically enriched compound of Formula V-1 or V-2-F by assymetrical reduction in the presence of a chiral organometallic catalyst.本文提供了制备具有I-014、I-020、I-064、I-074、I-082、I-089、I-090、I-095、I-171、I-181、I-184、I-186、I-189、I-191、I-192、I-193、I-205、I-206、I-208、I-211、I-212、I-213、I-220、I-229、I-231、I-233、I-234、I-246、I-251、I-258、I-259、I-262、I-263、I-285、I-323和I-400的立体富集化合物的方法。本文描述的化合物表现出作为杀虫剂的活性,并且在例如用于控制植物中由真菌病原体引起的真菌病害的方法中是有用的。一种首选的方法是通过在手性有机金属催化剂存在下进行不对称还原来制备具有V-1或V-2-F式的立体富集化合物。
-
Palladium-Catalyzed Carbonylation Reaction of Aryl Bromides with 2-Hydroxyacetophenones to Form Flavones作者:Xiao-Feng Wu、Helfried Neumann、Matthias BellerDOI:10.1002/chem.201202141日期:2012.10.1Flavone of the month: A general and efficient method for the palladium‐catalyzed carbonylative synthesis of flavones has been developed (see scheme). Starting from aryl bromides and 2‐hydroxyacetophenones, the corresponding flavones have been isolated in good yields.
-
Redox‐Neutral Coupling between Two C(sp <sup>3</sup> )−H Bonds Enabled by 1,4‐Palladium Shift for the Synthesis of Fused Heterocycles作者:Ronan Rocaboy、Ioannis Anastasiou、Olivier BaudoinDOI:10.1002/anie.201908460日期:2019.10.7secondary C-H bonds, which are adjacent to an oxygen or nitrogen atom on one side, and benzylic or adjacent to a carbonyl group on the other side. A variety of valuable fused heterocycles were obtained from easily accessible ortho-bromophenol and aniline precursors. The second C-H bond cleavage was successfully replaced with carbonyl insertion to generate other types of C(sp3 )-C(sp3 ) bonds.
-
[EN] MULTIPLE D2 A(NTA)GONISTS/H3 ANTAGONISTS FOR TREATMENT OF CNS-RELATED DISORDERS<br/>[FR] MULTIPLES A(NTA)GONISTES DE D2/ANTAGONISTES DE H3 POUR LE TRAITEMENT DE TROUBLES ASSOCIÉS AU SNC申请人:AAPA B V公开号:WO2015069110A1公开(公告)日:2015-05-14The present invention relates to compounds compound according to Formula (III); and pharmaceutically acceptable salts, hydrates and solvates thereof. These compounds have D2receptor antagonist/(partial) agonist effects and H3antagonistic effects, pharmaceutical compositions thereof, and methods of using them for application in the prophylaxis or treatment of CNS disorders.本发明涉及按照式(III)的化合物;以及其药学上可接受的盐、水合物和溶剂合物。这些化合物具有D2受体拮抗/(部分)激动剂效应和H3拮抗效应,以及其药物组合物,以及将其用于预防或治疗中枢神经系统疾病的方法。
-
Design, synthesis and biological evaluation of novel ring-opened cromakalim analogues with relaxant effects on vascular and respiratory smooth muscles and as stimulators of elastin synthesis作者:Mourad Bouhedja、Basile Peres、Wassim Fhayli、Zeinab Ghandour、Ahcène Boumendjel、Gilles Faury、Smail KheliliDOI:10.1016/j.ejmech.2017.12.071日期:2018.1vascular smooth muscle cells showed a strong stimulating effect on elastin synthesis, especially compound B16, which was more active at 20 μM than diazoxide, a reference ATP-sensitive potassium channel activator. Taken together, our results show that the N-methylation of the sulfonylurea moieties of ring-opened cromakalim analogues led to new compounds blocking calcium-gated channels, which had a major合成了两个新的含cromakalim的含磺酰脲部分的开环类似物系列(A系列:带有N-未甲基化的磺酰脲,B系列:带有N-甲基化的磺酰脲),并作为血管和呼吸道平滑肌(大鼠主动脉和气管,分别)。离体生物学评估表明,活性最高的化合物(系列B)对内皮完整的主动脉环和气管表现出显着的血管舒张活性。大多数B系列化合物的血管舒张活性(EC 50 <22μM)比参考化合物二氮嗪(EC 50)高 = 24μM)。有趣的是,与参考化合物克罗马卡林(EC 50 = 124μM),特别是化合物B4,B7和B16(EC 50 <10μM)相比,几种B系列测试化合物在气管上也表现出更强的松弛作用。与此相反,系列甲衍生物是在主动脉环差的活性(EC 50 > 57μM所有,并且EC 50 > 200μM为其中大部分),但它们中的一些显示出了一个有趣的放宽对气管(即作用A15和A33,EC 50 = 30μM)。
表征谱图
-
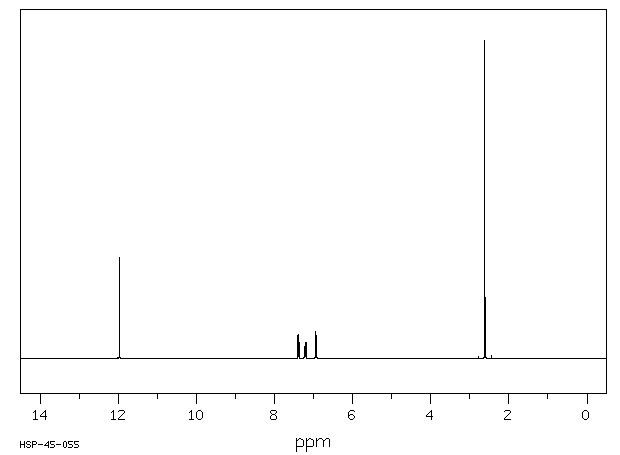
氢谱1HNMR
-
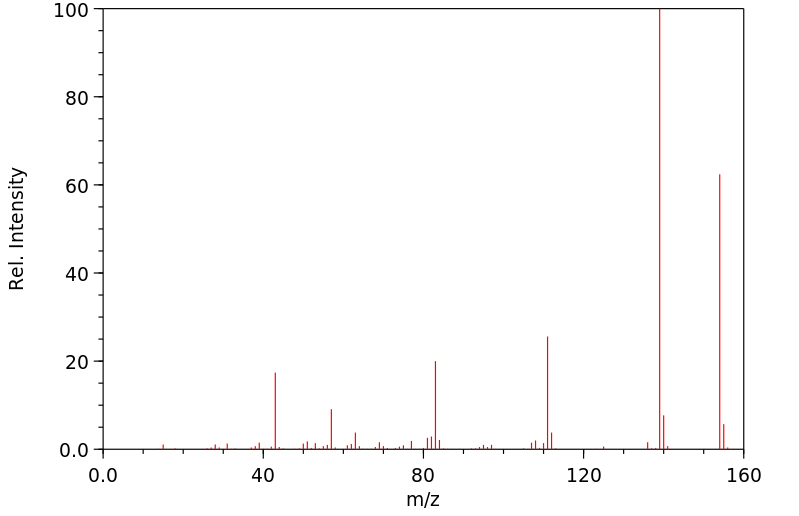
质谱MS
-
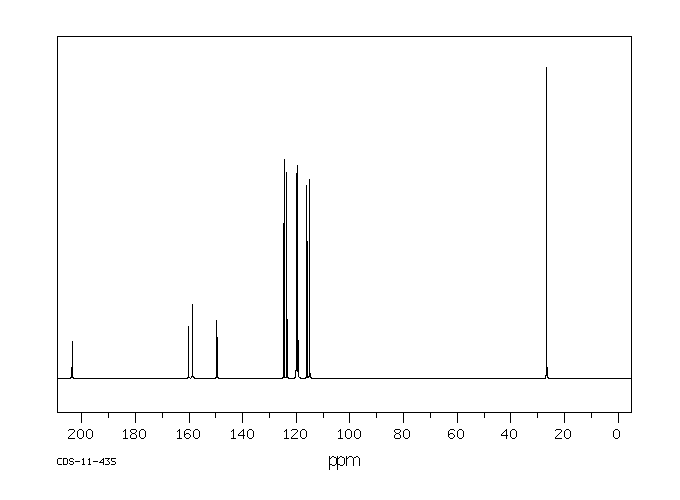
碳谱13CNMR
-
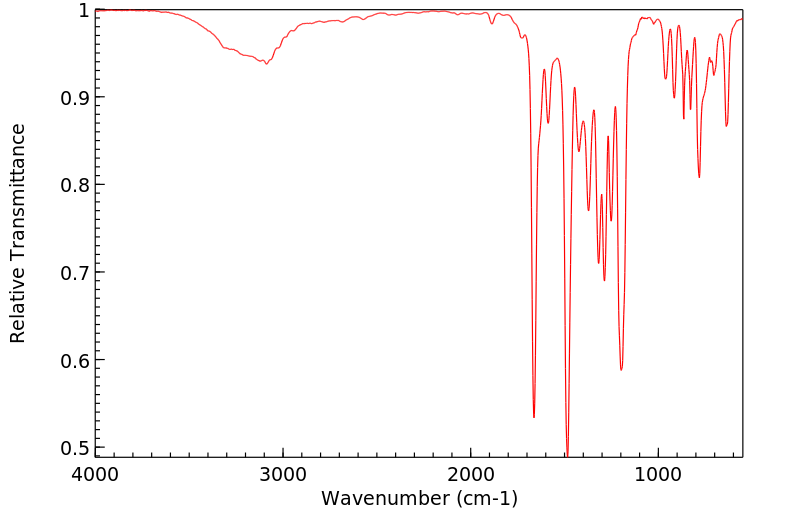
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷