N-甲基-2-硝基苯胺 | 612-28-2
中文名称
N-甲基-2-硝基苯胺
中文别名
N-甲邻硝苯胺
英文名称
2-nitro-N-methylaniline
英文别名
N-methyl-2-nitroaniline;N-methyl-o-nitroaniline;N-methyl-2-nitrobenzenamine;o-nitro-N-methylaminobenzene
CAS
612-28-2
化学式
C7H8N2O2
mdl
MFCD00007090
分子量
152.153
InChiKey
KFBOUJZFFJDYTA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35-38 °C (lit.)
-
沸点:294.61°C (rough estimate)
-
密度:1.2333 (estimate)
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:0.972
-
稳定性/保质期:
性质与稳定性:
常温常压下,该物质不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:57.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S36/37,S45
-
危险类别码:R23/24/25,R33
-
WGK Germany:3
-
RTECS号:BY5710000
-
包装等级:II
-
危险类别:6.1
-
危险品运输编号:2811
-
危险性防范说明:P280,P301+P310
-
危险性描述:H301+H311+H331,H373
-
储存条件:贮存: 将密封的药瓶放置于阴凉、干燥处,保存在密封的主容器中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: N-Methyl 2-nitroaniline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H311: Toxic in contact with skin
H331: Toxic if inhaled
H373: May cause damage to organs through prolonged or repeated exposure
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P311: Call a POISON CENTER or doctor/physician
Section 3. Composition/information on ingredients.
Ingredient name: N-Methyl 2-nitroaniline
CAS number: 612-28-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H8N2O2
Molecular weight: 152.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: II
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (N-Methyl 2-nitroaniline)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: N-Methyl 2-nitroaniline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H311: Toxic in contact with skin
H331: Toxic if inhaled
H373: May cause damage to organs through prolonged or repeated exposure
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P311: Call a POISON CENTER or doctor/physician
Section 3. Composition/information on ingredients.
Ingredient name: N-Methyl 2-nitroaniline
CAS number: 612-28-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H8N2O2
Molecular weight: 152.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: II
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (N-Methyl 2-nitroaniline)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基对硝基苯胺 N,N-dimethyl-2-nitro-benzenamine 610-17-3 C8H10N2O2 166.18 —— N,N'-bis(2-nitrophenyl)methanediamine 17465-20-2 C13H12N4O4 288.263 2-硝基苯胺 2-nitro-aniline 88-74-4 C6H6N2O2 138.126 2-硝基乙酰苯胺 o-nitroacetanilide 552-32-9 C8H8N2O3 180.163 1,2-二硝基苯 1,2-Dinitrobenzene 528-29-0 C6H4N2O4 168.109 N-甲基-N-(2-硝基苯基)乙酰胺 N-methyl-N-(2-nitrophenyl)acetamide 7418-33-9 C9H10N2O3 194.19 鄰硝苯甲醯胺苯 N-(2-nitrophenyl)benzanilide 728-90-5 C13H10N2O3 242.234 —— N-methyl-N-(2-nitrophenyl)methoxycarboxamide 93675-40-2 C9H10N2O4 210.189 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-2,6-二硝基苯胺 N-methyl-2,6-dinitroaniline 5910-19-0 C7H7N3O4 197.15 N-甲基-2,4-二硝基苯胺 N-methyl-2,4-dinitroaniline 2044-88-4 C7H7N3O4 197.15 4-溴-N-甲基-2-硝基苯胺 N-methyl-4-bromo-2-nitroaniline 53484-26-7 C7H7BrN2O2 231.049 3-溴-正甲基-2-硝基苯甲胺 3-bromo-N-methyl-2-nitroaniline 1150617-53-0 C7H7BrN2O2 231.049 —— N-methyl-2-nitro-N-nitrosoaniline 89937-90-6 C7H7N3O3 181.151 —— N-methyl-(2,2-dinitro)diphenylamine 119707-15-2 C13H11N3O4 273.248 —— N-methyl-N-(2-nitrophenyl)carbamoyl chloride 675839-78-8 C8H7ClN2O3 214.608 N-甲基-N-(2-硝基苯基)乙酰胺 N-methyl-N-(2-nitrophenyl)acetamide 7418-33-9 C9H10N2O3 194.19 —— 4,4’-methylene-2,2’-dinitro-bis(N-methyl-aniniline) 100974-85-4 C15H16N4O4 316.316 3-(甲基-(2-硝基苯基)氨基)丙酸 3-(methyl-(2-nitrophenyl)amino)propanoic acid 710349-37-4 C10H12N2O4 224.216 —— N-methyl-2-nitro-4-(propylthio)aniline 637331-60-3 C10H14N2O2S 226.299 —— N-methyl-N-(2-nitrophenyl)prop-2-enamide 169330-12-5 C10H10N2O3 206.201 —— N-methyl-N-(2-nitrophenyl)methoxycarboxamide 93675-40-2 C9H10N2O4 210.189 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:通过邻硝基硝基苯胺的膦介导的还原环化反应合成苯并咪唑摘要:加热邻硝基N-酰苯胺1 - 3和2-甲基- ñ - (3-硝基吡啶-2-基)丙酰胺(5)与4当量。的导致了2-取代的苯并咪唑的膦6 - 8和所述咪唑并[4,5- b ]吡啶10分别在45和85%之间的收率。用(EtO)3 P加热1会实现环化和N乙基化,从而生成1乙基苯并咪唑6b。N-新戊酰硝基苯胺2b的缓慢环化可分离出中间体膦亚胺11慢慢转化为1 H-苯并咪唑7b。的结构11,通过晶体结构分析确定。当N-甲基化的邻-硝基乙酰苯胺3环化成1,2-二甲基-1 H-苯并咪唑(8)时,2-甲基丙酰苯胺4被转化为1-甲基-3-(1-甲基乙基)-2 H-苯并咪唑‐2‐1(9)。DOI:10.1002/hlca.201100123
-
作为产物:描述:参考文献:名称:Steric-Hindrance-Induced Regio- and Chemoselective Oxidation of Aromatic Amines摘要:Unusual regio- and chemoselective oxidation of aromatic amines hindered with ortho substituents (except -NH2, -NHCH3, and -OH) to the corresponding nitro compounds is described by use of nonanebis(peroxoic acid). The mechanistic investigation for selective oxidation of amines ortho-substituted with -NH2 or -OH showed the involvement of H-bonding between the ortho hydrogen of the adjacent -XH group (where X = NH, NR, or O) and an oxygen atom from the diperoxy acid. Various mono- and diamines are oxidized into corresponding mononitro derivatives in high yield and purity without employing any protection strategies. The protocol was also found to successful on the gram scale.DOI:10.1021/acs.joc.5b00582
文献信息
-
Bicyclic Benzimidazole Compounds and Their Use as Metabotropic Glutamate Receptor Potentiators申请人:Egle Ian公开号:US20090192169A1公开(公告)日:2009-07-30Compounds of Formula I: wherein A, B, D, L, R 1 , R 2 , R 3 , R 4 , m, and n are as defined for Formula I in the description. The invention also relates to processes for the preparation of the compounds and to new intermediates employed in the preparation, pharmaceutical compositions containing the compounds, and to the use of the compounds in therapy.式I的化合物: 其中A、B、D、L、R1、R2、R3、R4、m和n的定义如描述中所定义。本发明还涉及制备这些化合物的方法,以及用于制备的新中间体,含有这些化合物的药物组合物,以及这些化合物在治疗中的应用。
-
Ru@PsIL-Catalyzed Synthesis of <i>N</i> -Formamides and Benzimidazole by using Carbon Dioxide and Dimethylamine Borane作者:Vitthal B. Saptal、Takehiko Sasaki、Bhalchandra M. BhanageDOI:10.1002/cctc.201800185日期:2018.6.21This work reports the synthesis and characterization of ruthenium nanoparticles (Ru NPs) supported on polymeric ionic liquids (PILs). This catalyst shows high catalytic activity towards the N‐formylation of amines and synthesis of benzimidazoles from 1,2‐diamines and carbon dioxide (CO2) by reductive dehydrogenation of dimethylamine borane. This methodology shows excellent functional group tolerance这项工作报告了载于聚合物离子液体(PIL)上的钌纳米颗粒(Ru NPs)的合成和表征。该催化剂对胺的N-甲酰化和由1,2-二胺和二氧化碳(CO 2)通过二甲胺硼烷的还原脱氢合成苯并咪唑具有很高的催化活性。该方法显示出优异的官能团耐受性,在合成N-甲酰胺和苯并咪唑的过程中具有广泛的底物范围。有趣的是,该协议还提供了串联减少2-硝基胺和CO 2的方法。合成苯并咪唑 提出了聚合物的离子液相起关键作用,例如帮助静电稳定纳米颗粒,提供离子环境并控制底物/试剂易于接近活性位。开发的方法利用CO 2作为C 1源和水/乙醇作为绿色溶剂系统。另外,发现该催化剂本质上是可再循环的,并且显示出连续五次循环运行而其活性没有明显损失。
-
Diversified facile synthesis of benzimidazoles, quinazolin-4(3H)-ones and 1,4-benzodiazepine-2,5-diones via palladium-catalyzed transfer hydrogenation/condensation cascade of nitro arenes under microwave irradiation作者:Kaicheng Zhu、Jian-Hong Hao、Cheng-Pan Zhang、Jiajun Zhang、Yiqing Feng、Hua-Li QinDOI:10.1039/c4ra15765f日期:——
An efficient methodology for diversified preparation of benzimidazole, quinazolin-4(3
H )-ones and 1,4-benzodiazepine-2,5-diones is established using a Pd-CTH/condensation cascade of nitro arenes in a TEA–formic acid mixture under microwave irradiation. -
An easy one-step photocatalytic synthesis of 1-aryl-2-alkylbenzimidazoles by platinum loaded TiO2 nanoparticles under UV and solar light作者:K. Selvam、M. SwaminathanDOI:10.1016/j.tetlet.2011.04.090日期:2011.6One-pot synthesis of disubstituted benzimidazoles from N-substituted 2-nitroanilines or 1,2-diamines with 3–12 nm-sized platinum particles loaded on the TiO2 using solar and UV-A light is described. 1-Aryl-2-alkylbenzimidazoles from 2-nitrodiphenylamines are formed by combined redox photocatalytic reaction, condensation and catalytic dehydrogenation on Pt-TiO2. In case of diamines, this reaction is
-
[EN] BENZOTHIADIAZINE COMPOUNDS<br/>[FR] COMPOSÉS BENZOTHIADIAZINE申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2017098421A1公开(公告)日:2017-06-15The invention is directed to substituted benzothiadiazine derivatives. Specifically, the invention is directed to compounds according to Formula (I):wherein R, R1, R2, R3, R4 and R5 are as defined herein. The compounds of the invention are inhibitors of CD73 and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases associated with CD73 inhibition, such as AIDS, autoimmune diseases, infections, atherosclerosis, and ischemia-reperfusion injury. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting CD73 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
表征谱图
-
氢谱1HNMR
-
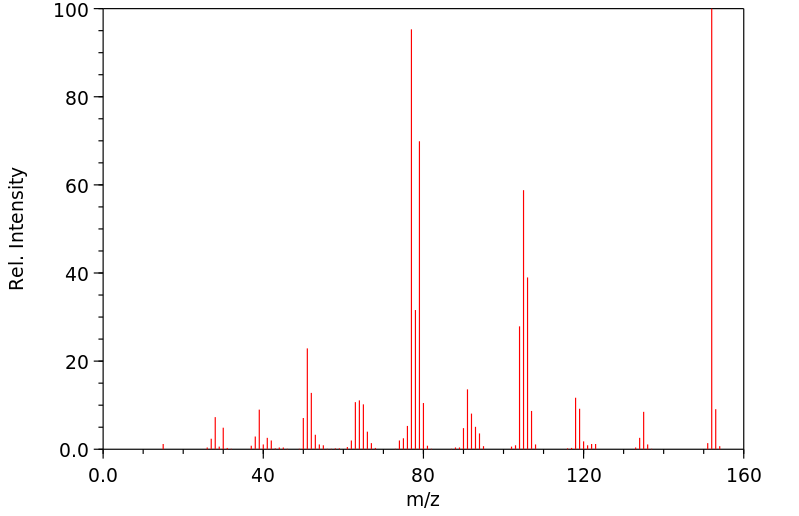
质谱MS
-
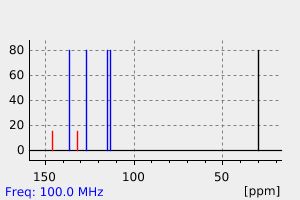
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫