N-苯基哌啶 | 4096-20-2
中文名称
N-苯基哌啶
中文别名
N-苯基六氢吡啶;1-苯基哌啶
英文名称
N-phenyl piperidine
英文别名
1-phenylpiperidine;4-phenylpiperidine;phenyl piperidine
CAS
4096-20-2
化学式
C11H15N
mdl
MFCD00129777
分子量
161.247
InChiKey
LLSKXGRDUPMXLC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:4.7°C
-
沸点:258°C(lit.)
-
密度:0.9944
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2933399090
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: N-Phenylpiperidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N-Phenylpiperidine
CAS number: 4096-20-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H15N
Molecular weight: 161.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: N-Phenylpiperidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: N-Phenylpiperidine
CAS number: 4096-20-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C11H15N
Molecular weight: 161.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(1-哌啶基)苯胺 4-(1-piperidino)aniline 2359-60-6 C11H16N2 176.261 5-(苯基氨基)戊烷-1-醇 5-(phenylamino)pentan-1-ol 40447-26-5 C11H17NO 179.262 —— N-(5-bromo-pentyl)-aniline 42331-03-3 C11H16BrN 242.159 —— N-phenylpiperidine N-oxide 19555-50-1 C11H15NO 177.246 N-苯基-2-哌啶酮 1-phenylpiperidin-2-one 4789-09-7 C11H13NO 175.23 —— 2-piperidin-1-yl-cycloheptatrienone 36359-79-2 C12H15NO 189.257 —— N-phenylglutarimide 5768-13-8 C11H11NO2 189.214 2-哌啶基-1-苯甲醛 2-(piperidin-1-yl)benzaldehyde 34595-26-1 C12H15NO 189.257 —— 5-(N-phenylamino)-1-pentyl acetate 13659-02-4 C13H19NO2 221.299 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(1-哌啶基)苯胺 4-(1-piperidino)aniline 2359-60-6 C11H16N2 176.261 1-(4-溴苯基)哌啶 N-(4-bromophenyl)piperidine 22148-20-5 C11H14BrN 240.143 —— N-(4-chlorophenyl)piperidine 40832-73-3 C11H14ClN 195.692 对哌啶苯酚 4-(piperidin-1-yl)phenol 24302-35-0 C11H15NO 177.246 1-(4-亚硝基-苯基)-哌啶 1-Piperidino-4-nitroso-benzol 52695-16-6 C11H14N2O 190.245 4-哌啶-1-基-苯甲醛 4-(piperidin-1-yl)benzaldehyde 10338-57-5 C12H15NO 189.257 —— 1-(4-isothiocyanatophenyl)piperidine 51317-65-8 C12H14N2S 218.323 (4-哌啶-1-苯基)甲醇 (4-(piperidin-1-yl)phenyl)methanol 677764-87-3 C12H17NO 191.273 1-[4-(4-哌啶-1-基苯基)苯基]哌啶 4,4'-di(piperidin-1-yl)biphenyl 279675-40-0 C22H28N2 320.478 —— 1-(4-thiocyanatophenyl)piperidine —— C12H14N2S 218.323 —— bis(4-(piperidin-1-yl)phenyl)methane 53926-62-8 C23H30N2 334.505 1-(4-硝基苯基)哌啶 1-(4-nitrophenyl)piperidine 6574-15-8 C11H14N2O2 206.244 —— 1-(2-chlorophenyl)piperidine 54121-55-0 C11H14ClN 195.692 —— 1-(2-bromophenyl)piperidine 82212-00-8 C11H14BrN 240.143 —— N-(5-iodopentyl)-N-phenylacetamide 1240498-24-1 C13H18INO 331.197 —— N-phenylpiperidine N-oxide 19555-50-1 C11H15NO 177.246 N-苯基-2-哌啶酮 1-phenylpiperidin-2-one 4789-09-7 C11H13NO 175.23 —— (4-chlorophenyl)-(4-piperidin-1-ylphenyl)diazene 89505-28-2 C17H18ClN3 299.803 —— N-(pent-4-en-1-yl)-N-phenylacetamide 1240498-25-2 C13H17NO 203.284 —— 4-(4-(piperidin-1-yl)phenyl)butan-2-one —— C15H21NO 231.338 —— 1-(2,4-dichlorophenyl)piperidine —— C11H13Cl2N 230.137 —— (E)-1-(4-cinnamylphenyl)piperidine —— C20H23N 277.409 —— 4-[(4-piperidin-1-ylphenyl)diazenyl]benzonitrile 89505-32-8 C18H18N4 290.368 —— [4-(piperidinyl)-benzenesulphonyl]chloride 125393-32-0 C11H14ClNO2S 259.757 —— 1-(4-(methylsulfonyl)phenyl)piperidine 150221-20-8 C12H17NO2S 239.338 —— 1-phenylpyrrolidine-2-carbaldehyde —— C11H13NO 175.23 1-苯基哌啶-2-甲腈 1-phenylpiperidine-2-carbonitrile 68078-10-4 C12H14N2 186.257 —— 4-(1-piperidinyl)benzenesulfonic acid —— C11H15NO3S 241.311 1-苯基-2-吡咯烷酮 1-phenylpyrrolidin-2-one 4641-57-0 C10H11NO 161.203 1-(4-碘苯基)-2-哌啶酮 1-(4-iodophenyl)piperidin-2-one 385425-15-0 C11H12INO 301.127 —— (4-nitrophenyl)-(4-piperidin-1-ylphenyl)diazene 89505-33-9 C17H18N4O2 310.356 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:N-苯基哌啶 在 tert-butylammonium hexafluorophosphate(V) 、 potassium carbonate 、 3-溴丙炔 作用下, 以 N-甲基吡咯烷酮 、 水 为溶剂, 反应 12.0h, 以81%的产率得到1-(4-溴苯基)哌啶参考文献:名称:使用炔丙基溴作为前所未有的溴源的 N,N-二取代苯胺的无金属电化学区域选择性芳族 C-H 溴化摘要:官能团的来源一直是化学合成中的重要因素。本文公开了一种在电化学条件下使用炔丙基溴作为非常规溴源的高区域选择性单溴化N,N-二取代苯胺的新方法。与其他溴源不同,它既不需要任何极化,也不需要任何活化剂。该反应在室温下顺利进行,无需使用任何金属催化剂或溴化物盐。对于未取代的苯胺,观察到区域选择性对位溴化,而间位和对位取代的苯胺均经历主要的邻位溴化,导致相应的N,N-二取代溴苯胺产率高。通过支持 B3LYP/6-31G DFT 计算描述了合理的机制,以解释相对能量驱动的过程。DOI:10.1016/j.tet.2022.132902
-
作为产物:描述:N-(Trifluoromethanesulfonyloxy)pyridinium triflate 在 platinum(IV) oxide 、 tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate 、 氢气 作用下, 以 乙醇 、 乙腈 为溶剂, 反应 3.5h, 生成 N-苯基哌啶参考文献:名称:芳烃CH氨基化的吡啶基自由基阳离子摘要:电子转移光催化使人们可以从选定的N-取代的吡啶鎓试剂中获得难以捉摸的N-吡啶基自由基阳离子。所得的(杂)芳烃的C(sp 2)-H功能化为开发有价值的胺化芳基骨架提供了多种中间体。包括第一个光谱学证据的自旋捕获的N-吡啶基自由基加合物的机理研究表明,SET触发了由可见光介导的N-X吡啶鎓试剂的伪介观裂解。DOI:10.1002/anie.201810261
-
作为试剂:描述:2,2,6,6-四甲基哌啶氧化物 、 过氧化二异丙苯 在 三乙烯二胺 、 iron(III) chloride 、 sodium metabisulfite 、 N-苯基哌啶 作用下, 以 乙腈 为溶剂, 反应 2.0h, 生成 1-methoxy-2,2,6,6-tetramethylpiperidine参考文献:名称:插入二氧化硫由饱和环胺合成β-甲基磺酰化的N-杂环摘要:FeCl 3催化的灭活环胺的C(sp 3)-H脱氢和C(sp 2)-H甲基磺酰化,并通过无机亚硫酸氢钠和过氧化二枯基的促进和参与,有效合成β-甲基磺酰化N-杂环。) 已经被开发出来。明显地,双官能DCP不仅充当促进脱氢的氧化剂,而且充当参与砜形成的甲基。通过该方案,以简便的一锅法获得了许多β-甲基磺酰化的四氢吡啶,四氢氮杂和吡咯。DOI:10.1021/acs.joc.0c02368
文献信息
-
Direct <i>para</i>-C–H heteroarylation of anilines with quinoxalinones by metal-free cross-dehydrogenative coupling under an aerobic atmosphere作者:Jun Xu、Lin Huang、Lei He、Chenfeng Liang、Yani Ouyang、Jiabin Shen、Min Jiang、Wanmei LiDOI:10.1039/d1gc01899j日期:——Herein, a green and efficient metal-free cross-dehydrogenative coupling (CDC) for the direct para-C–H heteroarylation of anilines with quinoxalinones has been described. This reaction is performed in H2O/DMSO (v/v = 2 : 1) using air as the sole oxidant. Various anilines (primary, secondary and tertiary amines) and quinoxalinones are well compatible, affording the corresponding products in moderate-to-good
-
[EN] AZADECALIN DERIVATIVES AS INHIBITORS OF HUMAN IMMUNODEFICIENCY VIRUS REPLICATION<br/>[FR] DÉRIVÉS D'AZADÉCALINE EN TANT QU'INHIBITEURS DE LA RÉPLICATION DU VIRUS DE L'IMMUNODÉFICIENCE HUMAINE申请人:VIIV HEALTHCARE UK (NO 5) LTD公开号:WO2018002848A1公开(公告)日:2018-01-04Compounds having drug and bio-affecting properties, their pharmaceutical compositions and methods of use are set forth. In particular, azadecaline derivatives that possess unique antiviral activity are provided as HIV maturation inhibitors, as represented by compounds of Formula (I). These compounds are useful for the treatment of HIV and AIDS.
-
Chemoselective Reductive Amination of Aldehydes and Ketones by Dibutylchlorotin Hydride-HMPA Complex作者:Toshihiro Suwa、Erika Sugiyama、Ikuya Shibata、Akio BabaDOI:10.1055/s-2000-6273日期:——Reductive amination of various aldehydes and ketones has been performed effectively by pentacoordinate chloro-substituted tin hydride complex, Bu2SnClH-HMPA. The tin reagent worked particularly well for the case using weakly basic aromatic amines as starting substrates. Stoichiometric amounts of a substrate and a reducing agent were adequate for the reaction. The Sn-Cl bond in the complex plays an
-
Porphyrin-Based Conjugated Microporous Polymer Tubes: Template-Free Synthesis and A Photocatalyst for Visible-Light-Driven Thiocyanation of Anilines作者:Pengfei Zhang、Yucheng Yin、Zhengxin Wang、Chunyang Yu、Yizhou Zhu、Deyue Yan、Weimin Liu、Yiyong MaiDOI:10.1021/acs.macromol.1c00190日期:2021.4.13photocurrent, compared to those of the irregular solid CMP counterpart. When serving as a metal-free photocatalyst for an undocumented visible-light-driven thiocyanation of anilines, CMP-1 exhibits excellent photocatalytic performance, with single chemoselectivity and high yields for the conversion of 25 types of anilines at ambient conditions. This study fills in the gap of the tubular morphological engineering
-
An Efficient and Simple Aqueous N-Heterocyclization of Aniline Derivatives: Microwave-Assisted Synthesis of <i>N</i>-Aryl Azacycloalkanes作者:Yuhong Ju、Rajender S. VarmaDOI:10.1021/ol050683t日期:2005.6.1[reaction: see text] An efficient and clean synthesis of N-aryl azacycloalkanes from alkyl dihalides and aniline derivatives has been achieved using microwave irradiation in an aqueous potassium carbonate medium. The phase separation can simplify the product isolation and reduce usage of volatile organic solvents.
表征谱图
-
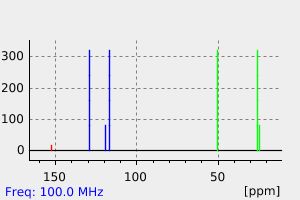
氢谱1HNMR
-
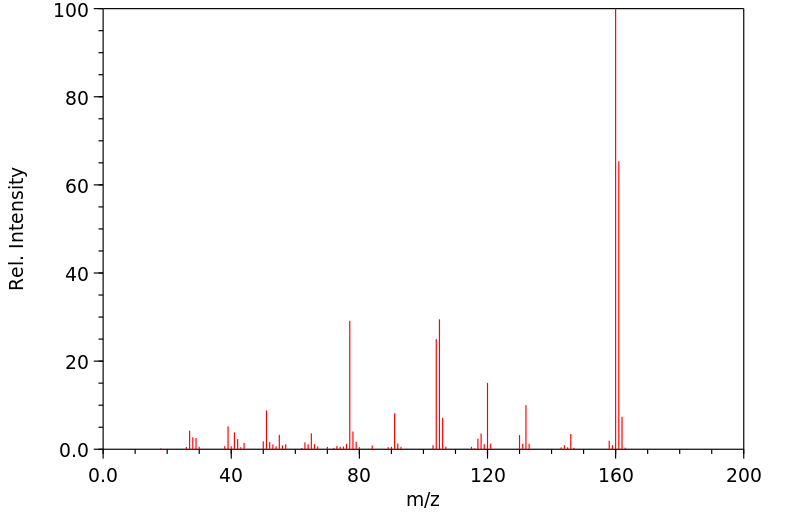
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺