乙醛 | 75-07-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-125 °C (lit.)
-
沸点:21 °C (lit.)
-
密度:0.785 g/mL at 25 °C (lit.)
-
蒸气密度:1.03 (vs air)
-
闪点:133 °F
-
溶解度:可溶于醇类
-
介电常数:21.8(5℃)
-
暴露限值:TLV-TWA 180 mg/m3 (100 ppm) (ACGIH), 360 mg/m3 (200 ppm) (NIOSH); STEL 270 mg/m3 (150 ppm); IDLH 10,000 ppm.
-
LogP:-0.16
-
物理描述:Acetaldehyde appears as a clear colorless liquid with a pungent choking odor. Flash point -36°F. Boiling point 69°F. Density 6.5 lb / gal. Vapors are heaver than air and irritate the mucous membranes and especially the eyes. Used to make other chemicals.
-
颜色/状态:Volatile liquid or gas
-
气味:Pungent, fruity odor
-
味道:Tart taste (fruits containing acetaldehyde before ripening)
-
蒸汽密度:1.52 (NTP, 1992) (Relative to Air)
-
蒸汽压力:902 mm Hg at 25 °C;758 mm Hg at 20 °C
-
亨利常数:6.67e-05 atm-m3/mole
-
大气OH速率常数:1.58e-11 cm3/molecule*sec
-
稳定性/保质期:
-
自燃温度:347 °F (175 °C)
-
分解:Decomposes above 400 °C to form ... methane & carbon monoxide.
-
粘度:0.253 mPa s at 9.5 °C; 0.21 mPa s at 20 °C
-
燃烧热:-1168.79 kJ/mol (liquid at constant pressure)
-
汽化热:25.73 kJ/mol at 20.2 °C
-
表面张力:21.2 mN/m at 20 °C (1.0 mN/m = 1.0 dyn/cm)
-
电离电位:10.22 eV
-
聚合:The substance may polymerize under the influence of acids, alkaline materials, such as sodium hydroxide, in the presence of trace metals (iron) with fire or explosion hazard. (From table)
-
气味阈值:Recognition in air= 2.1x10-1 ppm (chemically pure)
-
折光率:Index of refraction = 1.3316 at 20 °C
-
解离常数:pKa = 13.57 at 25 °C
-
保留指数:381;381;381;381;359;418;360.37;360.37;360.5;360.64;360.88;361.6;362.6;364;360;360;361;361;363;380;363;389;369;363;363;358;363;367;372;372;360;360;373;387.3;360;400;352;342;352;372;363;400;363;363;363;372
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:2,000 ppm
-
危险品标志:Xn
-
安全说明:S16,S26,S33,S36/37
-
危险类别码:R40,R36/37,R12
-
WGK Germany:2
-
海关编码:2912120000
-
危险品运输编号:UN 1198 3/PG 3
-
危险类别:3
-
RTECS号:LP8925000
-
包装等级:I
-
储存条件:储存于阴凉、通风的库房,远离火种、热源,库温不宜超过29℃。包装要求密封,不可与空气接触,并需与氧化剂、还原剂、酸类等分开存放,切忌混储。不宜大量储存或久存。 采用防爆型照明和通风设施,禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
无色、易燃、易挥发、易流动的液体,有辛辣刺激性气味。与水、乙醇、乙醚、苯、汽油、甲苯、二甲苯和丙酮混溶。
用途主要用于制备醋酸、醋酐、乙酸乙酯、丁醇、季戊四醇、三聚乙醛、3-羟基丁醛、三氯乙醛等产品。GB 2760—1996规定为暂时允许使用的食用香料,主要用于配制柑橘、苹果、奶油等型香精。乙醛的最大用户是乙酸行业,丁醇、辛醇过去也是乙醛的重要衍生产品,现在已基本为丙烯羰基合成法代替。乙醛的其他消费领域是生产季戊四醇、过乙酸、吡啶及其衍生物。国内生产的乙醛基本上都作为生产乙酸的中间体,只有少量用于生产季戊四醇、丁醇、三氯乙醛、三羟甲基丙烷等产品。
用途主要用于制备醋酸、醋酐、丁醛、辛醇、季戊四醇、三聚乙醛等重要的化工原料。用作还原剂及杀菌剂。
生产方法乙醛有多种生产方法:1. 乙烯直接氧化法,乙烯和氧气通过含有氯化钯、氯化铜、盐酸及水的催化剂,一步直接氧化合成粗乙醛,然后经蒸馏得成品;2. 乙醇氧化法,乙醇蒸气在300-480℃下,以银、铜或银-铜合金的网或粒作催化剂,由空气氧化脱氢制得乙醛;3. 乙炔直接水合法,乙炔和水在汞催化剂或非汞催化剂作用下,直接水合得到乙醛。因有汞害问题,已逐渐为他法取代;4. 乙醇脱氢法,在添加钴、铬、锌或其他化合物的铜催化剂作用下,乙醇脱氢生产乙醛;5. 饱和烃类氧化法。
生产方法由乙烯氧化而得。由乙醇进行气相氢化而得。由醋酸钙和甲酸钙同时干馏而得。由乙炔和水加合反应而得。
类别易燃液体
毒性分级中毒
急性毒性口服- 大鼠 LD50: 661 毫克/ 公斤;皮下- 小鼠 LD50: 560 毫克/ 公斤
刺激数据皮肤- 兔子 500 毫克 轻度;眼睛- 兔子 40 毫克 重度
爆炸物危险特性与空气混合可爆
可燃性危险特性遇明火、高温、氧化剂易燃;燃烧产生刺激烟雾
储运特性库房通风低温干燥;与氧化剂、酸类分开存放
灭火剂干粉、干砂、干石粉、二氧化碳、泡沫
职业标准TWA 120 毫克/立方米;STEL 180 毫克/立方米
上下游信息
反应信息
-
作为反应物:参考文献:名称:Process for the preparation of N-methylalkylamines摘要:一种制备CH.sub.3 --NH--CH.sub.2 --R式N-甲基烷胺的方法,其中R是具有1至3个碳原子的脂肪基,通过将R-CHO式醛与R'--NH.sub.2式胺反应以得到席夫碱,去除反应水,然后在氢化催化剂存在下将席夫碱与甲胺和氢反应。公开号:US05773658A1
-
作为产物:描述:N-亚硝基二乙胺 在 human cytochrome P450 2Ab 、 recombinant human cytochrome b5 from plasmid pSE420(Amp) 、 recombinant rat NADPH-P450 reductase 、 glucose-6-phosphate 、 nicotinamide adenine dinucleotide phosphate 、 1,2-二十二酰基-sn-glycero-3-胆碱磷酸 、 yeast glucose-6-phosphate dehydrogenase 作用下, 反应 0.25h, 生成 乙醛参考文献:名称:人类细胞色素 P450 2A6 氧化 N-亚硝基烷基胺:连续氧化成醛和羧酸以及反应步骤分析。摘要:细胞色素 P450 (P450) 2A6 将亚硝胺,包括 N,N-二甲基亚硝胺 (DMN) 和 N,N-二乙基亚硝胺 (DEN) 激活为烷基重氮氧化物(它们是 DNA 烷基化剂)和醛(来自 DMN 和 CH(3 )CHO 来自 DEN)。DMN 的 N-脱烷基化具有很高的固有动力学氘同位素效应((D)k(app) 约 10),这在各种竞争性和非竞争性实验中得到了高度表达。DEN 的 (D)k(app) 约为 3,在非竞争性实验中未表达。DMN 和 DEN 也分别被氧化为 HCO(2)H 和 CH(3)CO(2)H。在这两种情况下都没有观察到滞后,考虑到测量 DMN 和 DEN 氧化成醛和醛氧化成羧酸的 k(cat) 和 K(m) 参数,这是出乎意料的。光谱分析没有表明醛对 P450 2A6 有很强的亲和力,但脉冲追踪实验表明,在 DMN 和 DEN 氧化成羧酸的过程中,与添加的(未标记的)醛只有有限的交换。在DOI:10.1074/jbc.m109.088039
-
作为试剂:描述:4-乙基苯甲腈 在 N-羟基邻苯二甲酰亚胺 、 氧气 、 乙醛 作用下, 以 乙腈 为溶剂, 40.0 ℃ 、101.33 kPa 条件下, 反应 6.0h, 生成 对氰基苯乙酮 、 4-(1-hydroperoxyethyl)benzonitrile参考文献:名称:温和条件下取代乙苯的选择性催化好氧氧化摘要:乙苯在温和条件下,通过由醛和N-羟基邻苯二甲酰亚胺(NHPI)组成的无金属催化体系,以高选择性氧化为相应的氢过氧化物(PEHP )。该过程是通过自由基机制通过原位生成邻苯二甲酰亚胺-N发生的-氧基(PINO)自由基。该协议已成功应用于各种取代的乙苯(ETB)。在几对ETB上进行的竞争性实验显示出明显的极性效应,这证明了PINO至少在低转化率下作为真正的氢提取物发挥了关键作用。在较高的转化率下,由PEHP形成的高反应性OH自由基可减少选定的ETB对的反应性差异。对反应机理的研究,包括对醛和催化剂百分含量的研究,以及温度和浓度的影响,可以使最终的PEHPs产品获得良好的收率。DOI:10.1016/j.molcata.2011.12.009
文献信息
-
Plant Growth Regulator Daminozide Is a Selective Inhibitor of Human KDM2/7 Histone Demethylases作者:Nathan R. Rose、Esther C. Y. Woon、Anthony Tumber、Louise J. Walport、Rasheduzzaman Chowdhury、Xuan Shirley Li、Oliver N. F. King、Clarisse Lejeune、Stanley S. Ng、Tobias Krojer、Mun Chiang Chan、Anna M. Rydzik、Richard J. Hopkinson、Ka Hing Che、Michelle Daniel、Claire Strain-Damerell、Carina Gileadi、Grazyna Kochan、Ivanhoe K. H. Leung、James Dunford、Kar Kheng Yeoh、Peter J. Ratcliffe、Nicola Burgess-Brown、Frank von Delft、Susanne Muller、Brian Marsden、Paul E. Brennan、Michael A. McDonough、Udo Oppermann、Robert J. Klose、Christopher J. Schofield、Akane KawamuraDOI:10.1021/jm300677j日期:2012.7.26N-demethylation of Nε-methyl lysine residues in histones and are current therapeutic targets. A set of human 2-oxoglutarate analogues were screened using a unified assay platform for JmjC demethylases and related oxygenases. Results led to the finding that daminozide (N-(dimethylamino)succinamic acid, 160 Da), a plant growth regulator, selectively inhibits the KDM2/7 JmjC subfamily. Kinetic and crystallographic
-
C5 설폰 화합물, 그의 제조방법 및, 이를 이용한 크로세틴 디니트릴 제조방법 및 그의 용도申请人:Myongji University Industry and Academia Cooperation Foundation 명지대학교 산학협력단(220050139720) BRN ▼135-82-11060公开号:KR20150115997A公开(公告)日:2015-10-15본 발명은 신규의 C5 설폰 화합물, 그 제조방법 및 이를 이용한 크로세틴 디니트릴 제조방법에 관한 것으로, 더욱 상세하게는 하이드라존 보호기를 갖는 신규의 C5 설폰 화합물, 그 제조방법과 이를 이용하여 크로세틴 디니트릴 화합물을 효율적으로 제조할 수 있는 방법에 관한 것이다. 본 발명에 따른 신규의 설폰 화합물은 안정하고, 결정성이 좋아 분리 정제가 용이하고, 이중 결합 형성시 E-구조 형성이 용이하다. 또한 상기 설폰 화합물을 이용하여 크로세틴 디니트릴을 합성하는 경우 결합 반응의 부산물인 설핀 산의 제거가 용이하기 때문에 순수한 최종 생성물을 효율적으로 얻을 수 있다. 본 발명에 따른 크로세틴 디니트릴 화합물은 카로틴 화합물의 일종으로 양 말단에 니트릴기를 함유하여 다양한 반응성을 기대할 수 있다. 또한 양 말단에 포함된 질소원자는 금속에 대한 친화력이 우수하기 때문에 금속 표면에 자기 조립이 가능하다. 아울러 카로틴의 일반적인 항산화 기능을 나타낼 수 있고 유기 분자 도선 등 전기 전자 재료로서도 사용이 가능하다.本发明涉及一种新的C5硫腈化合物,其制备方法以及用于制备克罗塞汀二腈的方法,更详细地说,涉及一种具有羟甲酰保护基的新型C5硫腈化合物,其制备方法以及利用该化合物有效制备克罗塞汀二腈化合物的方法。根据本发明的新型硫腈化合物是稳定的,结晶性好,易于分离纯化,并且在形成双键时易于形成E-构型。此外,利用上述硫腈化合物合成克罗塞汀二腈时,由于易于去除偶联反应的副产物丙烯酸,因此可以有效地获得纯净的最终产物。根据本发明的克罗塞汀二腈化合物是一种胡萝卜素化合物,其末端含有腈基,可以期望具有多种反应性。此外,末端的氮原子对金属具有优良的亲和力,因此可以在金属表面上进行自组装。此外,它还可以表现出胡萝卜素的一般抗氧化功能,并且可以用作有机分子导线等电子电气材料。
-
Phenanthroline-7-one derivatives and their therapeutic uses申请人:Laboratoire L. Lafon公开号:US06809096B1公开(公告)日:2004-10-26A pharmaceutical composition including an efficient amount of a compound selected among the compounds of formulae (I) and (Ia). The compounds have interesting cytotoxic properties leading to a therapeutic use as antitumoral medicines.一种包括在式(I)和(Ia)的化合物中选择的有效量化合物的制药组合物。这些化合物具有引导其作为抗肿瘤药物的治疗用途的有趣细胞毒性特性。
-
A Novel, One-Pot Synthesis of α-<i>C</i>-Cyanohydrazines in the Presence of Lithium Perchlorate/Diethylether Solution (5.0 M)作者:Akbar Heydari、Robabe Baharfar、Mohsen Rezaie、Saied M. AslanzadehDOI:10.1246/cl.2002.368日期:2002.3Condensation of N,N-dimethylhydrazine, an aldehyde in lithium perchlorate/diethylether solution (5.0 M) gave N,N-dimethylhydrazone, which were treated with trimethylsilyl-cyanide to afford α-C-cyanohydrazine. These compounds are important precursors of nitrogen-substituted reagents.
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
表征谱图
-
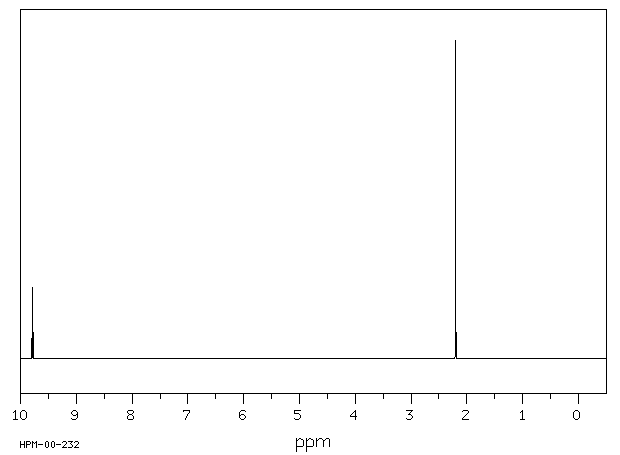
氢谱1HNMR
-
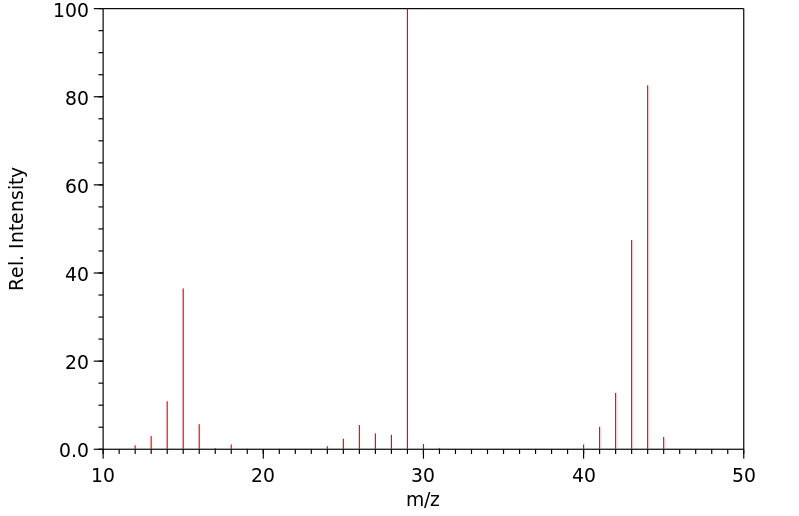
质谱MS
-
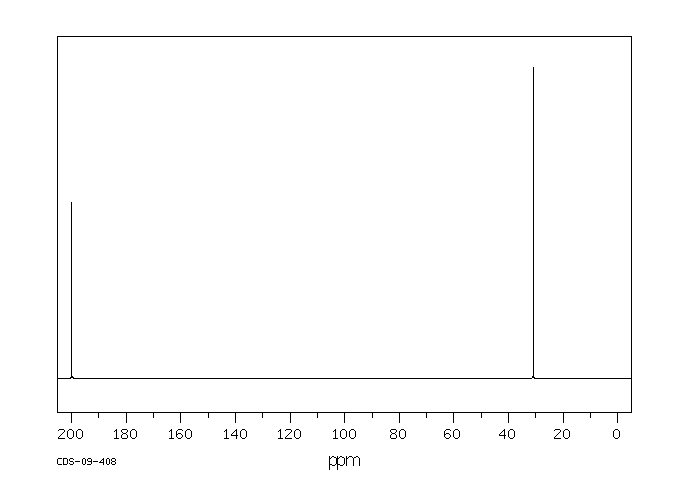
碳谱13CNMR
-
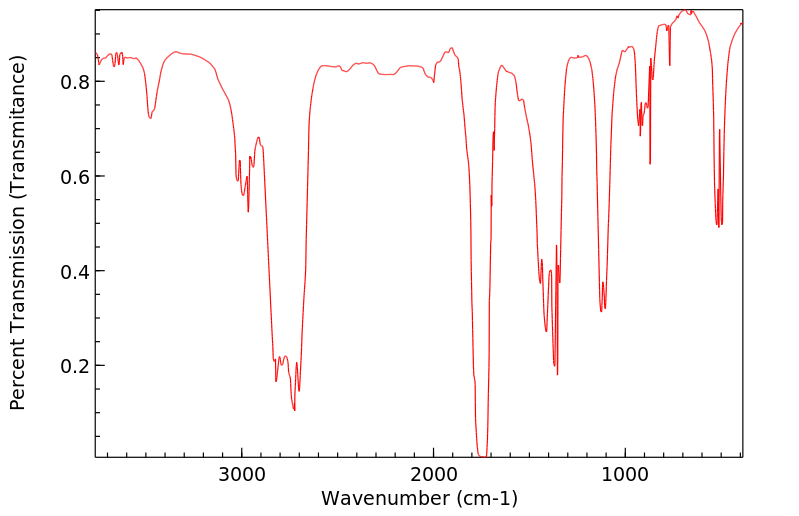
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息