1-萘甲基胺 | 118-31-0
中文名称
1-萘甲基胺
中文别名
1-萘甲胺
英文名称
(naphth-1-yl)methylamine
英文别名
1-Naphthylmethylamine;naphthalen-1-ylmethanamine;1-naphthalenemethylamine;1-naphthylmethanamine;naphthyl methyl amine
CAS
118-31-0
化学式
C11H11N
mdl
MFCD00004048
分子量
157.215
InChiKey
NVSYANRBXPURRQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:262-269 °C
-
沸点:290-293 °C (lit.)
-
密度:1.073 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:与乙醇、乙醚和二硫化碳混溶。
-
稳定性/保质期:
常温常压下保持稳定,应避免与强氧化剂或空气直接接触。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2921499090
-
危险品运输编号:2735
-
危险类别:8
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:储存于阴凉、通风的库房中。远离火源和热源,与氧化剂分开存放,切忌混储。应配备相应品种和数量的消防器材。储区内应备有合适的材料以处理泄漏情况。
SDS
| Name: | 1-Naphthalenemethylamine Material Safety Data Sheet |
| Synonym: | 1-(Aminomethyl)naphthalen |
| CAS: | 118-31-0 |
Synonym:1-(Aminomethyl)naphthalen
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 118-31-0 | 1-Naphthalenemethylamine | 204-244-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Will burn if involved in a fire.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Keep under a nitrogen blanket.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 118-31-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 290 - 293 deg C @760mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.073
Molecular Formula: C11H11N
Molecular Weight: 157.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents, acids, acid anhydrides, chloroformates, acid chlorides.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 118-31-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Naphthalenemethylamine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 118-31-0: No information available.
Canada
CAS# 118-31-0 is listed on Canada's NDSL List.
CAS# 118-31-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 118-31-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-1-萘甲胺 N-methyl-1-naphthalenemethylamine 14489-75-9 C12H13N 171.242 1-(叠氮甲基)萘 1-(azidomethyl)naphthalene 55210-79-2 C11H9N3 183.213 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 萘-1-硫代甲酰胺 1-naphthalenecarbothioamide 20300-10-1 C11H9NS 187.265 萘-1-甲酰胺 1-naphthylcarboxamide 2243-81-4 C11H9NO 171.199 (1-萘基)甲基氨基甲酸 (1-naphthyl)methylcarbamic acid 477710-75-1 C12H11NO2 201.225 萘-2-甲酰胺 2-naphthamide 2243-82-5 C11H9NO 171.199 1-萘甲醛 1-naphthaldehyde 66-77-3 C11H8O 156.184 氰基萘 1-Cyanonaphthalene 86-53-3 C11H7N 153.183 N-去甲基特比萘芬 (E)-6,6-dimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine 99473-11-7 C20H23N 277.409 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2 (氯甲基)萘 1-Chloromethylnaphthalene 86-52-2 C11H9Cl 176.645 1-溴甲基萘 2-(bromomethyl)naphthalene 3163-27-7 C11H9Br 221.096 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-1-萘甲胺 N-methyl-1-naphthalenemethylamine 14489-75-9 C12H13N 171.242 1-(叠氮甲基)萘 1-(azidomethyl)naphthalene 55210-79-2 C11H9N3 183.213 N,N-二甲基-1-(萘-1-基)甲胺 N,N-dimethyl(naphthalen-1-yl)methanamine 16413-71-1 C13H15N 185.269 N-(1-萘基甲基)乙胺 N-ethyl-1-naphthalenemethanamine 14489-76-0 C13H15N 185.269 —— N-(naphthalen-1-ylmethyl)formamide 1219505-32-4 C12H11NO 185.225 —— 1-isocyanatomethyl-naphthalene 61924-27-4 C12H9NO 183.21 —— 1-(naphthalen-1-yl)-N-(naphthalen-1-ylmethyl)methanamine 5798-49-2 C22H19N 297.4 苄萘-1-甲胺 N-benzyl-1-(naphthalen-1-yl)methanamine 14393-12-5 C18H17N 247.34 1-萘甲基异硫氰酸 1-(isothiocyanatomethyl)naphthalene 17112-82-2 C12H9NS 199.276 —— [1]naphthylmethyl-[2]naphthylmethyl-amine 47292-14-8 C22H19N 297.4 —— 9-(1-naphthylmethylaminomethyl)anthracene 190431-40-4 C26H21N 347.459 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 (1-萘甲基)胍 (1-naphthylmethyl)guanidine 5696-79-7 C12H13N3 199.255 萘-1-甲酰胺 1-naphthylcarboxamide 2243-81-4 C11H9NO 171.199 —— 1-(naphthalen-1-ylmethyl)thiourea —— C12H12N2S 216.307 —— (E)-3-chloro-N-(naphthalen-1-ylmethyl)prop-2-en-1-amine —— C14H14ClN 231.725 N-(1-萘基甲基)-乙酰胺 N-(1-naphthylmethyl)acetamide 19351-91-8 C13H13NO 199.252 (1-萘基)甲基氨基甲酸 (1-naphthyl)methylcarbamic acid 477710-75-1 C12H11NO2 201.225 —— 1-(4-chlorophenyl)-N-(naphthalen-1-ylmethyl)methanamine 14393-17-0 C18H16ClN 281.785 —— 2-chloro-2-fluoro-N-(1-naphthylmethyl)ethanamine 958643-16-8 C13H13ClFN 237.704 —— N-(4-Brombenzyl)-1-naphthylmethyl-amin 14393-18-1 C18H16BrN 326.236 —— 1,3-bis(naphthalen-1-ylmethyl)urea 83263-17-6 C23H20N2O 340.425 —— N-(1-naphthylmethyl)acrylamide 89919-19-7 C14H13NO 211.263 —— methyl N-[(1-naphthyl)methyl]carbamate 92277-77-5 C13H13NO2 215.252 —— N-(naphthalene-1-ylmethyl)-2-bromoacetamide 256656-69-6 C13H12BrNO 278.148 —— 2-chloro-N-naphthalen-1-yl-methyl-acetamide 204253-07-6 C13H12ClNO 233.697 —— N-(1-naphthalenylmethylene)-1-naphthalenemethanamine —— C22H17N 295.384 —— N-(1-methylnaphthyl)-N'-(n-dodecyl) carbodiimide 1263270-95-6 C24H34N2 350.547 —— 2-chloro-2,2-difluoro-N-(1-naphthylmethyl)ethanamine 958643-31-7 C13H12ClF2N 255.695 N-[(1-萘基)甲基]苯胺 N-(naphthalen-1-ylmethyl)aniline 7182-94-7 C17H15N 233.313 —— N-(1-naphthylmethyl)pyrrolidine 91462-80-5 C15H17N 211.307 1-萘甲醛 1-naphthaldehyde 66-77-3 C11H8O 156.184 氰基萘 1-Cyanonaphthalene 86-53-3 C11H7N 153.183 N-去甲基特比萘芬 (E)-6,6-dimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine 99473-11-7 C20H23N 277.409 —— (E)-methyl 3-(1-naphthylamino)acrylate 1416084-47-3 C15H15NO2 241.29 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2 —— trans-N-(1-naphthylmethyl)-5-trimethylsilyl-pent-2-en-4-ynyl-1-amine 84213-97-8 C19H23NSi 293.484 —— (E)-N-(naphthalen-1-ylmethyl)-3-phenylprop-2-en-1-amine 92610-10-1 C20H19N 273.378 —— 2-cyano-N-(naphthalen-1-ylmethyl)acetamide 566926-34-9 C14H12N2O 224.262 —— (naphthalen-1-ylmethyl-amino)-acetic acid ethyl ester 163116-06-1 C15H17NO2 243.305 —— N-(3-phenyl-2-propenylidene)-1-naphthalenemethanamine 98977-78-7 C20H17N 271.362 (4-甲氧苯基)-N-(萘-1-基甲基)甲胺 ML133 185669-79-8 C19H19NO 277.366 —— <α-Naphthyl-methyl>-urethan 4096-83-7 C14H15NO2 229.279 —— 3-[(Naphthalen-1-ylmethyl)-amino]-propionic acid ethyl ester 131436-70-9 C16H19NO2 257.332 —— N-(naphthalen-1-ylmethyl)benzamide 32280-54-9 C18H15NO 261.323 —— N-(2-hydroxy-ethyl)-N'-[1]naphthylmethyl-urea 101938-67-4 C14H16N2O2 244.293 —— N-(naphthalen-1-ylmethyl)cyclohexanamine 14489-84-0 C17H21N 239.36 —— 1-(naphthalene-1-yl)but-3-en-1-amine —— C14H15N 197.28 —— 2-Azido-N-naphthalen-1-ylmethyl-acetamide 595606-99-8 C13H12N4O 240.264 —— Biphenyl-4-ylmethyl-naphthalen-1-ylmethyl-amine 185669-81-2 C24H21N 323.437 —— 1-(3-butylthioureidomethyl)naphthalene —— C16H20N2S 272.414 萘-1-羧酸二甲基酰胺 N,N-dimethyl-1-naphthamide 3815-24-5 C13H13NO 199.252 —— 2-chloro-2-fluoro-N-(1-naphthylmethyl)acetamide 958643-12-4 C13H11ClFNO 251.688 —— N-Naphthalen-1-ylmethyl-benzamidine 198065-85-9 C18H16N2 260.338 —— cyclopropanecarboxylic acid (naphthalen-1-ylmethyl)-amide 1268340-91-5 C15H15NO 225.29 —— N-Naphthalen-1-ylmethyl-oxalamic acid 81682-61-3 C13H11NO3 229.235 —— N-methyl-N-<2(E),4(E)-nonadienyl>-1-naphthalinmethanamin 92525-78-5 C21H27N 293.452 —— 1-(naphthalen-2-yl)-N-(naphthalen-2-ylmethyl)methanimine —— C22H17N 295.384 —— tert-butyl 3-((naphthalen-1-ylmethyl)amino)propanoate 1422663-23-7 C18H23NO2 285.386 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:参考文献:名称:六氟异丙醇中分子间反应中甲醛介导的烷基胺氢化物释放摘要:烷基胺自发释放氢阴离子的能力通常受到限制,除非在特定的分子内反应设置下。在此,我们证明这种反应活性可以通过在六氟异丙醇(HFIP)溶剂中用甲醛进行简单处理来释放,从而在温和条件下实现烷基胺的各种分子间氢化物转移反应。除了小分子的转化之外,这些反应还可以对复杂肽进行独特的后期修饰。机理研究发现,这些分子间氢化物转移过程的关键在于溶剂介导的大环过渡态的调节构象,其中 HFIP 分子的聚集体充当灵巧的质子穿梭机。重要的是,氮的孤电子对与胺的αC-H键的反键轨道之间的负超共轭在C-H活化中起着关键作用,促进其氢化物释放。DOI:10.1021/jacs.3c12215
-
作为产物:描述:1-萘甲醇 在 potassium tert-butylate 、 氨 作用下, 以 甲苯 为溶剂, 150.0 ℃ 、700.01 kPa 条件下, 反应 24.0h, 以59%的产率得到1-萘甲基胺参考文献:名称:用于胺和氨与醇的一般和选择性N-烷基化的可重复使用的Co纳米颗粒摘要:报道了一种通用的钴催化胺与醇的N-烷基化反应,利用氢方法制备不同种类的胺。这种转化的最佳催化剂是通过热解特定的模板材料来制备的,该模板材料是通过混合钴盐、氮配体和胶态二氧化硅原位生成的,然后去除二氧化硅。应用这种新型Co纳米粒子基材料,>100伯胺、仲胺和叔胺(包括N-甲胺)和选定的药物分子可以从廉价且容易获得的醇和胺或氨开始方便地制备。DOI:10.1039/d1sc05913k
-
作为试剂:参考文献:名称:New 8-(3-amino-piperidin-1-yl)-7-(but-2-ynyl)-xanthines, the preparation thereof and their use as pharmaceutical compositions摘要:该申请涉及一般式的新取代黄嘌呤,其中R1和R2如权利要求1至11中定义的,其互变异构体、对映体、非对映体、其混合物及其盐,具有有价值的药理特性,特别是对酶二肽基肽酶-IV(DPP-IV)的抑制作用。公开号:US20060058323A1
文献信息
-
Dehydrogenation of Primary Alkyl Azides to Nitriles Catalyzed by Pincer Iridium/Ruthenium Complexes作者:Lan Gan、Xiangqing Jia、Huaquan Fang、Guixia Liu、Zheng HuangDOI:10.1002/cctc.202000260日期:2020.7.21Pincer metal complexes exhibit superior catalytic activity in the dehydrogenation of plain alkanes, but find limited application in the dehydrogenation of functionalized organic molecules. Starting from easily accessible primary alkyl azides, here we report an efficient dehydrogenation of azides to nitriles using pincer iridium or ruthenium complexes as the catalysts. This method offers a route to
-
NNP-Type Pincer Imidazolylphosphine Ruthenium Complexes: Efficient Base-Free Hydrogenation of Aromatic and Aliphatic Nitriles under Mild Conditions作者:Rosa Adam、Elisabetta Alberico、Wolfgang Baumann、Hans-Joachim Drexler、Ralf Jackstell、Henrik Junge、Matthias BellerDOI:10.1002/chem.201504709日期:2016.3.24seven novel NImNHP‐type pincer imidazolylphosphine ruthenium complexes has been synthesized and fully characterized. The use of hydrogenation of benzonitrile as a benchmark test identified [RuHCl(CO)(NImNHPtBu)] as the most active catalyst. With its stable Ru−BH4 analogue, in which chloride is replaced by BH4, a broad range of (hetero)aromatic and aliphatic nitriles, including industrially interesting
-
Efficient Assembly of Iminodicarboxamides by a “Truly” Four-Component Reaction作者:Kareem Khoury、Mantosh K. Sinha、Tadamichi Nagashima、Eberhardt Herdtweck、Alexander DömlingDOI:10.1002/anie.201205366日期:2012.10.8Mix and match: Similar to a galaxy consisting of millions of stars, a multicomponent reaction (MCR) system can result in millions of compounds. The MCR of α‐amino acids, oxo components, isocyanides, and amines leads to numerous and diverse compounds, thus having enormous potential for drug discovery or catalyst screening.
-
Structure–Activity Relationship in Pyrazolo[4,3-<i>c</i>]pyridines, First Inhibitors of PEX14–PEX5 Protein–Protein Interaction with Trypanocidal Activity作者:Maciej Dawidowski、Vishal C. Kalel、Valeria Napolitano、Roberto Fino、Kenji Schorpp、Leonidas Emmanouilidis、Dominik Lenhart、Michael Ostertag、Marcel Kaiser、Marta Kolonko、Bettina Tippler、Wolfgang Schliebs、Grzegorz Dubin、Pascal Mäser、Igor V. Tetko、Kamyar Hadian、Oliver Plettenburg、Ralf Erdmann、Michael Sattler、Grzegorz M. PopowiczDOI:10.1021/acs.jmedchem.9b01876日期:2020.1.23PEX14-PEX5 protein-protein interaction (PPI) is an attractive way to affect multiple metabolic pathways. Herein, we have used structure-guided computational screening and optimization to develop the first line of compounds that inhibit PEX14-PEX5 PPI. The optimization was driven by several X-ray structures, NMR binding data, and molecular dynamics simulations. Importantly, the developed compounds show锥虫的原生生物是导致一系列毁灭性传染病的病原体。针对锥虫的可用化学疗法的范围是有限的,并且现有疗法部分无效并且会引起严重的不良反应。PEX14-PEX5复合物的形成对于将蛋白质导入寄生虫的糖体至关重要。这种运输对寄生虫的代谢至关重要,失败会导致糖体酶的错误定位,并对寄生虫造成致命的后果。因此,抑制PEX14-PEX5蛋白-蛋白相互作用(PPI)是影响多种代谢途径的一种有吸引力的方法。在本文中,我们已使用结构指导的计算筛选和优化方法来开发抑制PEX14-PEX5 PPI的第一类化合物。优化是由几个X射线结构,NMR结合数据,和分子动力学模拟。重要的是,已开发的化合物对锥虫具有显着的细胞活性,包括人类病原体布鲁氏冈比亚锥虫和克氏锥虫寄生虫。
-
Nickel-Catalyzed Amination of Aryl Thioethers: A Combined Synthetic and Mechanistic Study作者:Alessandro Bismuto、Tristan Delcaillau、Patrick Müller、Bill MorandiDOI:10.1021/acscatal.0c00393日期:2020.4.17and kinetically competent catalysts for this transformation. The fleeting transmetalation intermediate has been successfully synthesized through an alternative synthetic organometallic pathway at lower temperature, allowing for in situ NMR study of the C–N bond reductive elimination step. This study addresses key factors governing the mechanism of the nickel-catalyzed Buchwald–Hartwig amination process
表征谱图
-
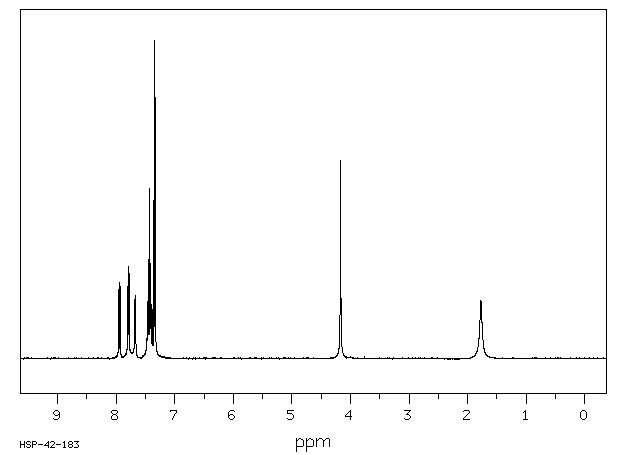
氢谱1HNMR
-
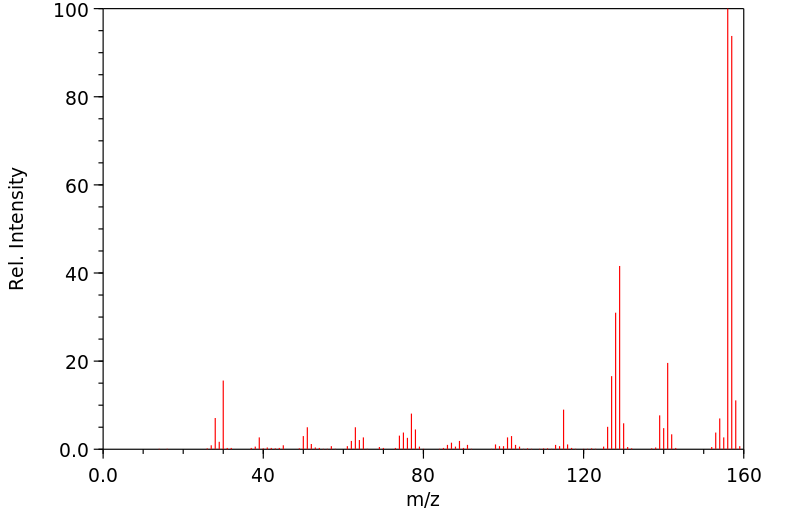
质谱MS
-
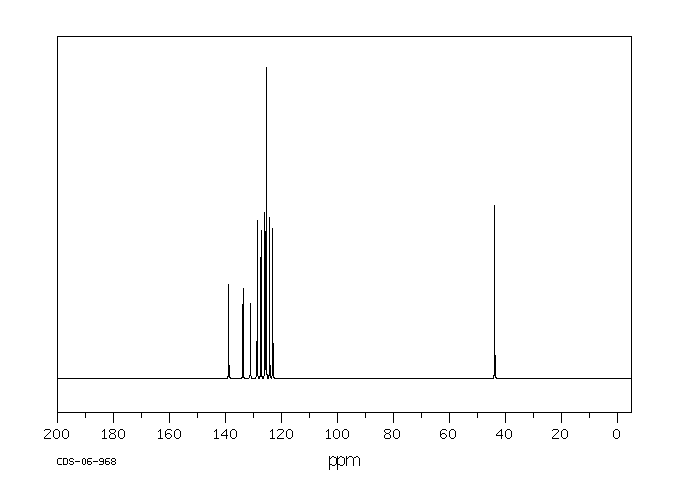
碳谱13CNMR
-
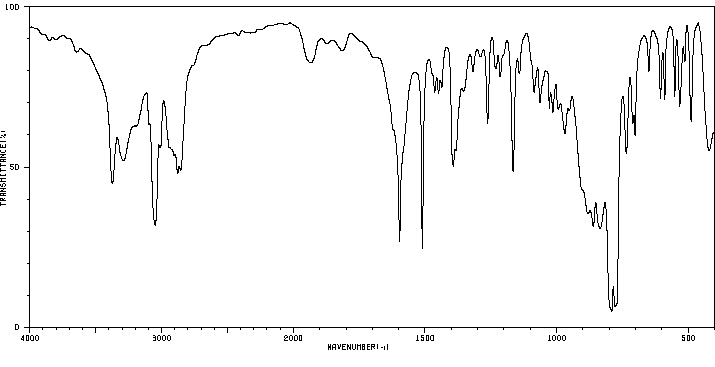
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮