2,4-二硝基苯肼盐酸盐 | 55907-61-4
中文名称
2,4-二硝基苯肼盐酸盐
中文别名
2,4-二硝基苯肼盐酸盐[用于高效液相色谱标记]
英文名称
2,4-dinitrophenylhydrazine hydrochloride
英文别名
(2,4-Dinitrophenyl)hydrazine;hydron;chloride;(2,4-dinitrophenyl)hydrazine;hydron;chloride
CAS
55907-61-4
化学式
C6H7N4O4*Cl
mdl
——
分子量
234.599
InChiKey
PUYFAZQODQZOFK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160 °C
-
溶解度:在热稀盐酸中几乎透明
计算性质
-
辛醇/水分配系数(LogP):1.21
-
重原子数:15
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:130
-
氢给体数:3
-
氢受体数:6
安全信息
-
危险等级:4.1
-
海关编码:2928000090
-
危险品运输编号:UN 1325
-
储存条件:存放于惰性气体中,避免接触空气和湿气(特别是吸湿性物质)。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2,4-Dinitrophenylhydrazine Hydrochloride
[for HPLC Labeling]
Portland OR
2,4-Dinitrophenylhydrazine Hydrochloride (for HPLC
Synonym
labeling)
Chemical Formula C6H6N4O4.HCl
55907-61-4
CAS Number
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2,4-Dinitrophenylhydrazine Hydrochloride 55907-61-4 Min. 98.0 Not available. Not available.
[for HPLC Labeling]
(HPLC,T)
Section III. Hazards Identification
No specific information is available in our data base regarding the toxic effects of this material for humans. However,
Acute Health Effects
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. DO NOT use an eye ointment. Flush eyes with running water for a minimum of 15
minutes, occasionally lifting the upper and lower eyelids. Seek medical attention. Treat symptomatically and supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin with running
water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the irritated skin with
an emollient. Seek medical attention. Treat symptomatically and supportively. Wash any contaminated clothing before
reusing.
If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
Inhalation
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was
ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible, show
the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Not available.
Flammable. Auto-Ignition
Flammability
Flash Points Flammable Limits Not available.
Not available.
These products are toxic carbon oxides (CO, CO2), nitrogen oxides (NO, NO2), halogenated compounds.
Combustion Products
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO2, water spray or foam.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Continued on Next Page
2,4-Dinitrophenylhydrazine Hydrochloride
[for HPLC Labeling]
Section VI. Accidental Release Measures
Spill Cleanup Flammable solid.
In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Use a
Instructions
shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any contaminated
surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage FLAMMABLE SOLID. MAY REACT VIOLENTLY AND/OR EVOLVE HEAT UPON EXPOSURE TO SHOCK, HEAT, AND
FRICTION. Reactive with strong oxidizers; may be ignited by heat, sparks, or flames. Vapors may travel to source of
Information
ignition and flash back. Tightly seal container and store in a cool place. Closed containers may explode from heat of a fire.
Empty containers may pose a fire risk. Evaporate residue under a fume hood if possible. Ground all equipment containing
material. Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the
container and store in a dry, cool place. Avoid excessive heat and light. DO NOT breathe dust. In case of insufficient
ventilation, wear suitable respiratory equipment. If you feel unwell, seek medical attention and show the label when possible.
Treat symptomatically and supportively. Avoid contact with skin and eyes.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
Engineering Controls
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
Personal Protection
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this
product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Powder. Solubility
Physical state @ 20°C Not available.
Not available.
Specific Gravity
234.6
Molecular Weight Partition Coefficient Not available.
Boiling Point Not available. Vapor Pressure Not available.
Not available. Not available.
Melting Point Vapor Density
Not available. Volatility Not available.
Refractive Index
Not available.
Critical Temperature Not available. Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
Not available.
RTECS Number
Eye contact. Inhalation. Ingestion.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling this
compound.
Continued on Next Page
2,4-Dinitrophenylhydrazine Hydrochloride
[for HPLC Labeling]
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of this substance.
Section XIV. Transport Information
DOT CLASS 4.1: Flammable solid.
DOT Classification
PIN Number
Proper Shipping Name Flammable solid, organic, n.o.s.
Packing Group (PG) II
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not controlled under WHMIS (Canada).
(Canada)
EINECS Number (EEC) 259-888-5
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:CoPc-catalyzed selective radical arylation of anilines with arylhydrazines for synthesis of 2-aminobiaryls摘要:描述了CoPc催化的选择性自由基芳基化反应,将苯胺与芳基肼反应,生成中等到良好产率的2-氨基二芳基化合物。DOI:10.1039/c4ob00798k
-
作为产物:描述:参考文献:名称:Design, Synthesis, Crystal Structures, and Insecticidal Activities of Eight-Membered Azabridge Neonicotinoid Analogues摘要:Three series of novel azabridge neonicotinoid analogues were designed and synthesized, which were constructed by starting material A, glutaraldehyde, and primary amine hydrochlorides (aliphatic amines, phenylhydrazines, and anilines). Most of the eight-membered azabridge compounds presented higher insecticidal activities than oxabridged compound B against cowpea aphid (Aphis craccivora) and brown planthopper (Nilaparvata lugens). Compared with imidacloprid, some azabridged compounds exhibited excellent insecticidal activity against brown planthopper. The crystal structures and bioassay indicated that changing bridge atoms from O to N could lead to entirely different conformations, which might be the important influential factor of the bioactivities.DOI:10.1021/jf4046683
文献信息
-
An Iodide-Mediated Transition-Metal-Free Strategy towards Unsymmetrical Diaryl Sulfides via Arylhydrazines and Thiols作者:Farnaz Jafarpour、Mohammad Asadpour、Meysam Azizzade、Mehran Ghasemi、Saideh Rajai-DaryasareiDOI:10.1055/s-0039-1690757日期:2020.3A mild, scalable iodine-mediated oxidative cross-coupling reaction of arylhydrazines and thiols for construction of thioethers (sulfides) in the absence of any transition metals or photocatalysts is disclosed. A variety of unsymmetrical diaryl sulfides with broad substrate scope both on thiols and hydrazines were synthesized in high yields in water at room temperature. Furthermore, to demonstrate the
-
[EN] DETECTION OF CHOLESTEROL OZONATION PRODUCTS<br/>[FR] DETECTION DE PRODUITS D'OZONATION DU CHOLESTEROL申请人:SCRIPPS RESEARCH INST公开号:WO2005023831A1公开(公告)日:2005-03-17The invention relates to detection of cholesterol ozonation products that are generated by atherosclerotic plaque material, and to methods of detecting vascular conditions that relate to the accumulation and oxidation of cholesterol.
-
Synthesis of novel dihydrotriazine derivatives bearing 1,3-diaryl pyrazole moieties as potential antibacterial agents作者:Tian-Yi Zhang、Chang-Ji Zheng、Jie Wu、Liang-Peng Sun、Hu-Ri PiaoDOI:10.1016/j.bmcl.2019.02.033日期:2019.5Three novel series of dihydrotriazine derivatives bearing 1,3-diaryl pyrazole moieties were designed, synthesized and evaluated in terms of their antibacterial and antifungal activities. Most of the synthesized compounds showed potent inhibition of several Gram-positive bacterial strains (including multidrug-resistant clinical isolates) and Gram-negative bacterial strains with minimum inhibitory concentration设计,合成和评估了三个带有1,3-二芳基吡唑部分的二氢三嗪衍生物系列,并对其抗菌和抗真菌活性进行了评估。大多数合成的化合物均显示出对几种革兰氏阳性细菌菌株(包括耐多药临床分离株)和革兰氏阴性细菌菌株的有效抑制作用,其最低抑菌浓度值在1-64 µg / mL的范围内。化合物4b和4c对革兰氏阳性菌(金黄色葡萄球菌4220,MRSA 3167,QRSA 3519)和革兰氏阴性菌(大肠杆菌1924)表现出最强的抑制活性,最小抑菌浓度值为1或2 µg /毫升 与以前的研究相比,这些化合物表现出广谱的抑制活性。化合物4a,4b,在L02细胞中评估4c和11n。体外酶研究表明,化合物4c通过抑制DHFR发挥其抗菌活性。
-
Oxidative behaviours and relative reactivities of some α-hydroxy acids towards bromate ion in hydrochloric acid medium作者:Kalyan Kali Sen Gupta、Amalendu Banerjee、Hrishikesh ChatterjeeDOI:10.1016/s0040-4020(01)89029-3日期:——parameters of the reactions have been compared. The plausible mechanism of the oxidation process has been suggested. The reactivity of the α-hydroxy acids towards bromate ion are as follows: 9-hydroxy-9-carboxy fluorene > atrolactic acid > mandelic acid > benzilic acid > α-hydroxyisobutyric acid > lactic acid > glycolic acid.
-
Synthesis and selective human monoamine oxidase inhibition of 3-carbonyl, 3-acyl, and 3-carboxyhydrazido coumarin derivatives作者:Daniela Secci、Simone Carradori、Adriana Bolasco、Paola Chimenti、Matilde Yáñez、Francesco Ortuso、Stefano AlcaroDOI:10.1016/j.ejmech.2011.07.017日期:2011.10Several 3-carbonyl (1–26), 3-acyl (27–52), and 3-carboxyhydrazido (53–58) coumarins have been synthesized in high yields (72–99%) and tested in vitro for their human monoamine oxidase A and B (hMAO-A and hMAO-B) inhibitory activity. Different substituents on the coumarin nucleus were evaluated for their effect on biological activity and isoform selectivity. Substitution at position C7 of the 3-ethyl
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
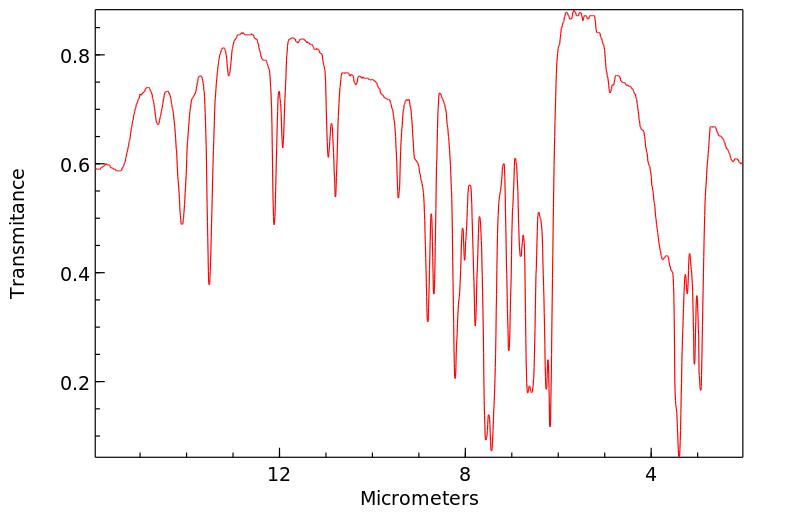
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫