3,5-二碘邻羟基苯醛 | 2631-77-8
中文名称
3,5-二碘邻羟基苯醛
中文别名
3,5-二碘水杨醛
英文名称
3,5-diodosalicylaldehyde
英文别名
3,5-diiodosalicylaldehyde;2-hydroxy-3,5-diiodobenzaldehyde;3,5-diiodo-2-hydroxybenzaldehyde;3,5-diidosalicylaldehyde;3,5-diiodosalicylic aldehyde
CAS
2631-77-8
化学式
C7H4I2O2
mdl
MFCD00003321
分子量
373.917
InChiKey
MYWSBJKVOUZCIA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:109-110 °C (lit.)
-
沸点:304.6±42.0 °C(Predicted)
-
密度:2.602±0.06 g/cm3(Predicted)
-
最大波长(λmax):360nm(MCH)(lit.)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S26,S37/39
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2913000090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封在阴凉干燥的环境中。
SDS
上下游信息
反应信息
-
作为反应物:描述:3,5-二碘邻羟基苯醛 在 sodium tetrahydroborate 作用下, 以 水 为溶剂, 生成 2,4-diiodo-6-((pyridine-2-ylmethylamino)methyl)phenol参考文献:名称:不对称卤素取代的 [NNʹO] 配体的铂 (II) 和铜 (II) 配合物:合成、表征、结构研究和抗增殖活性摘要:为了更好地了解结构、卤素取代、金属离子和配体柔韧性对抗增殖活性的影响,得到了2,4-X 1 ,X 2 -6-的8个Cu(II)配合物和8个Pt(II)配合物。 ((吡啶-2-基甲基氨基)甲基)苯酚和2,4-X 1 ,X 2-6-((吡啶-2-基甲基氨基)乙基)苯酚(其中 X 是 Cl、Br 或 I)配体。采用多种技术对化合物进行表征,如FT-IR、NMR、元素分析和单晶X射线衍射(SCXRD)。X 射线结构表明,配体分别在 Cu(II) 和 Pt(II) 配合物中充当二齿和三齿供体。这种结构上的差异是由于在制备复合物时使用或不使用碱所致。此外,Cl 2 - H 2 L 1与 CuCl 2 ·2H 2 O 的络合产生两种不同类型的结构:聚合物(Cl 2 -H 2 L 1 -Cu聚合物)和二聚体(Cl 2 -H 2 L 1 -Cu二聚体),根据晶体颜色。此外,铂配合物的1 H NMR 光谱显示DOI:10.1016/j.bioorg.2021.105556
-
作为产物:参考文献:名称:碘氯化吡啶鎓:一种用于碘化羟基化芳族酮和醛的有效试剂摘要:用碘氯化吡啶鎓 (PyICI) 对几种反应性芳族化合物(如羟基取代的苯乙酮和醛)进行直接碘化反应顺利进行,以良好至优异的收率得到相应的芳族碘化物。碘氯化吡啶鎓已被发现是一种有效的固体碘化试剂,没有有害影响,并且可以安全处理。DOI:10.1002/jccs.200800130
文献信息
-
4-Aminobenzoic Acid Derivatives: Converting Folate Precursor to Antimicrobial and Cytotoxic Agents作者:Martin Krátký、Klára Konečná、Jiří Janoušek、Michaela Brablíková、Ondřej Janďourek、František Trejtnar、Jiřina Stolaříková、Jarmila VinšováDOI:10.3390/biom10010009日期:——of non-toxic PABA resulted in constitution of antibacterial activity including inhibition of methicillin-resistant Staphylococcus aureus (minimum inhibitory concentrations, MIC, from 15.62 µM), moderate antimycobacterial activity (MIC ≥ 62.5 µM) and potent broad-spectrum antifungal properties (MIC of ≥ 7.81 µM). Some of the Schiff bases also exhibited notable cytotoxicity for cancer HepG2 cell line4-氨基苯甲酸(PABA)是许多人类病原体的必需营养素,但对人类来说却是可有可无的,其衍生物表现出多种生物活性。在这项研究中,我们使用分子杂交方法组合了两种药效团:这种维生素样分子和各种芳香醛,包括水杨醛和5-硝基糠醛,通过亚胺键一步反应。筛选所得希夫碱作为潜在的抗菌剂和细胞毒剂。对无毒的 PABA 进行简单的化学修饰,使其具有抗菌活性,包括抑制耐甲氧西林金黄色葡萄球菌(最低抑菌浓度,MIC,从 15.62 µM 起)、中等抗分枝杆菌活性(MIC ≥ 62.5 µM)和强效广谱抗真菌活性特性(MIC ≥ 7.81 µM)。一些希夫碱还对癌症 HepG2 细胞系表现出显着的细胞毒性 (IC50 ≥ 15.0 µM)。对于用于 PABA 衍生化的醛,可以调整特定的活性并获得具有良好生物活性的衍生物。
-
Gold-Catalyzed Tandem Intramolecular Heterocyclization/Petasis-Ferrier Rearrangement of 2-(Prop-2-ynyloxy)benzaldehydes as an Expedient Route to Benzo[b]oxepin-3(2 H)-ones作者:Ella Min Ling Sze、Weidong Rao、Ming Joo Koh、Philip Wai Hong ChanDOI:10.1002/chem.201003096日期:2011.2.1The golden ring: A synthetic approach to benzo[b]oxepin‐3(2 H)‐ones by heterocyclization/Petasis–Ferrier rearrangement of 2‐(prop‐2‐ynyloxy)benzaldehydes is reported. Uniquely, the ring formation was found to only proceed efficiently in the presence of a gold catalyst. Substitution at the position ortho to the ethereal group on the salicylaldehyde ring was shown to dramatically enhance reactivity (see
-
Copper-Catalyzed Asymmetric Oxidation of Sulfides作者:Graham E. O’Mahony、Alan Ford、Anita R. MaguireDOI:10.1021/jo2026178日期:2012.4.6Copper-catalyzed asymmetric sulfoxidation of aryl benzyl and aryl alkyl sulfides, using aqueous hydrogen peroxide as the oxidant, has been investigated. A relationship between the steric effects of the sulfide substituents and the enantioselectivity of the oxidation has been observed, with up to 93% ee for 2-naphthylmethyl phenyl sulfoxide, in modest yield in this instance (up to 30%). The influence
-
Investigation of steric and electronic effects in the copper-catalysed asymmetric oxidation of sulfides作者:Graham E. O'Mahony、Kevin S. Eccles、Robin E. Morrison、Alan Ford、Simon E. Lawrence、Anita R. MaguireDOI:10.1016/j.tet.2013.08.063日期:2013.11in the copper-catalysed asymmetric oxidation of aryl benzyl, aryl alkyl and alkyl benzyl sulfides have been investigated. The presence of an aryl group directly attached to the sulfur is essential to afford sulfoxides with high enantioselectivities, with up to 97% ee for 2-naphthyl benzyl sulfoxide, the highest enantioselectivity achieved to date for copper-catalysed asymmetric sulfoxidation. In contrast
-
Optimizing the structure of (salicylideneamino)benzoic acids: Towards selective antifungal and anti-staphylococcal agents作者:Martin Krátký、Klára Konečná、Kateřina Brokešová、Jana Maixnerová、František Trejtnar、Jarmila VinšováDOI:10.1016/j.ejps.2021.105732日期:2021.4human pathogenic bacteria and fungi has become a global health problem. Based on previous reports of 4-(salicylideneamino)benzoic acids, we designed, synthesised and evaluated their me-too analogues as potential antimicrobial agents. Forty imines derived from substituted salicylaldehydes and aminobenzoic acids, 4-aminobenzoic acid esters and 4-amino-N-phenylbenzamide were designed using molecular hybridization人类病原性细菌和真菌的抵抗力增强已成为全球性的健康问题。根据先前关于4-(水杨亚氨基氨基)苯甲酸的报道,我们设计,合成和评估了它们的中型类似物作为潜在的抗菌剂。40个亚胺衍生自取代的水杨醛和氨基苯甲酸,4-氨基苯甲酸酯和4-氨基-N使用分子杂交和前药策略设计了苯基苯甲酰胺。以高收率合成目标化合物并通过光谱方法表征。他们针对一组革兰氏阳性和革兰氏阴性细菌,分枝杆菌,酵母菌和霉菌进行了调查。测试了活性最高的亚胺,以确定它们在HepG2细胞中的细胞毒性和选择性。基于二卤代水杨醛的衍生物显示出有效的广谱抗菌特性,特别是针对革兰氏阳性细菌,包括耐甲氧西林的金黄色葡萄球菌(最低抑菌浓度,MIC从7.81 µM)和粪肠球菌(MIC≥15.62µM),酵母菌(MIC从MIC 7.81 µM)和叉毛癣菌模具(MIC≥3.90µM)。4-[(2-羟基-3,5-二碘亚苄基)氨基]苯甲酸甲酯4h表现出优异的体外活
表征谱图
-
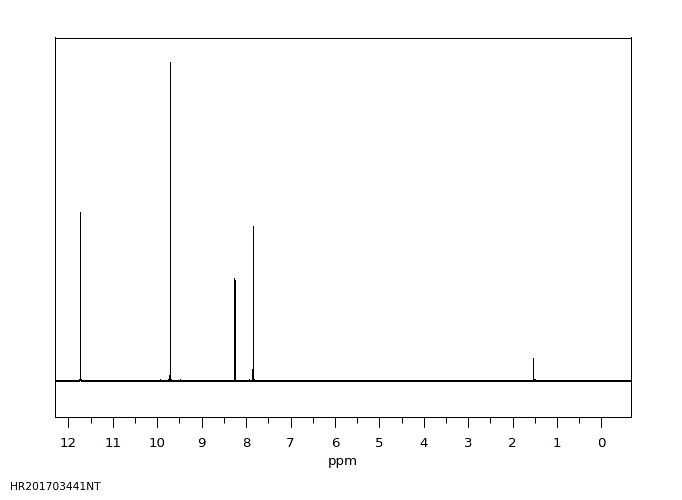
氢谱1HNMR
-
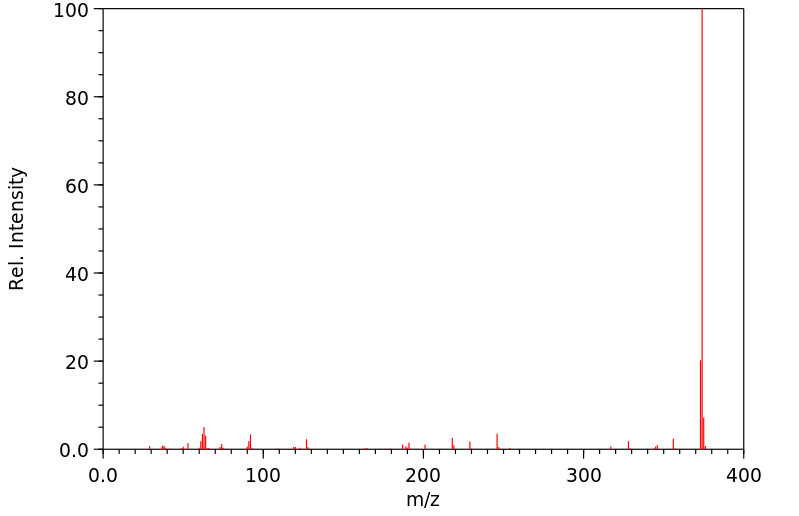
质谱MS
-
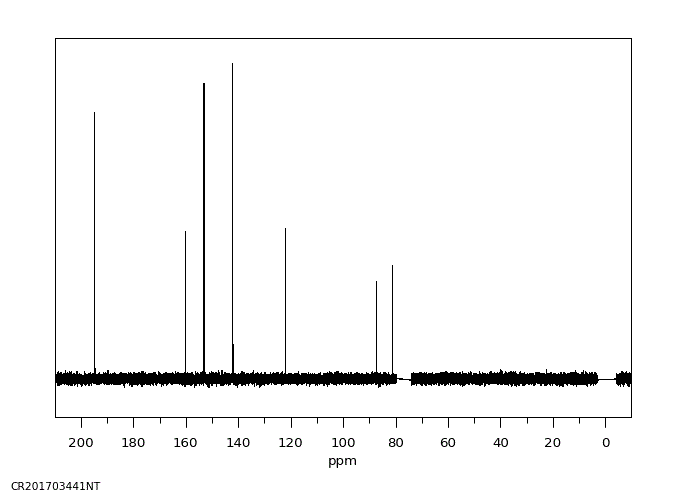
碳谱13CNMR
-
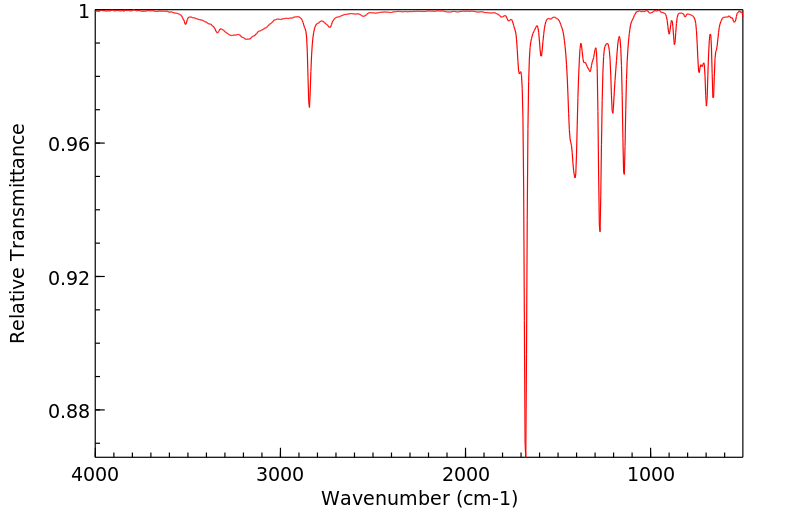
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷