磷酸吡哆醛 | 54-47-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-143 °C
-
沸点:565.7±60.0 °C(Predicted)
-
密度:1.638±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(轻微加热)、甲醇(轻微)、水(轻微)
-
LogP:-1.921 (est)
-
物理描述:Solid
-
碰撞截面:151.4 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-1.1
-
重原子数:16
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:117
-
氢给体数:3
-
氢受体数:7
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2936250000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:本品应密封存放在4℃、干燥避光的环境中。
SDS
| Name: | Pyridoxal 5-Phosphate 99% (Titr.) Material Safety Data Sheet |
| Synonym: | 3-Hydroxy-2-Methyl-5-(Phosphonoxy)-4-Pyridinecarboxaldehyde; 1-Iodohexadecane |
| CAS: | 54-47-7 |
Synonym:3-Hydroxy-2-Methyl-5-(Phosphonoxy)-4-Pyridinecarboxaldehyde; 1-Iodohexadecane
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 54-47-7 | 4-Pyridinecarboxaldehyde,3-Hydroxy-2-M | 99 | 200-208-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Light sensitive.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. Low hazard for usual industrial handling.
Inhalation:
May cause respiratory tract irritation. Low hazard for usual industrial handling.
Chronic:
None
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. If irritation develops, get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Get medical aid if irritation or symptoms occur.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. No special precautions indicated. Store protected from light.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 54-47-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H10NO6P
Molecular Weight: 247.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, light, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide, oxides of potassium.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 54-47-7: UV1207000 LD50/LC50:
CAS# 54-47-7: Oral, mouse: LD50 = 4640 mg/kg; Oral, rat: LD50 = 5900 mg/kg.
Carcinogenicity:
4-Pyridinecarboxaldehyde,3-Hydroxy-2-Methyl-5-(Phosphonooxy) - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 54-47-7: 1
Canada
CAS# 54-47-7 is listed on Canada's DSL List.
CAS# 54-47-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 54-47-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
磷酸吡哆醛是一种有机化合物,分子式C8H10NO6P,由维生素B6与磷酸结合形成,包括吡哆醛、吡哆胺和吡哆醇。在体内以磷酸酯形式存在,并且其中的磷酸吡哆醛和磷酸吡哆胺可以互相转变,皆为活性型。磷酸吡哆醛是维生素B6的活性形式,在催化多种反应中起到重要作用。
生化作用—辅酶
磷酸吡哆醛广泛参与转氨基作用、α-脱羧作用、β-脱羧作用、β-消除作用、γ-消除作用、消旋作用及羟醛反应等。在所有转氨基作用以及某些氨基酸的脱羧与脱氨反应中,它作为辅酶发挥作用。
磷酸吡哆醛通过其醛基与特定赖氨酸基团形成希夫碱键(内部醛亚胺),参与催化反应。例如,在丝氨酸脱水酶和GDP-4-酮-6-脱氧甘露糖-3-脱水酶(ColD)的反应中,磷酸吡哆醛与谷氨酸起反应,生成磷酸吡哆胺(PMP)。随后,磷酸吡哆胺转移氮到特定底物上,形成氨基糖。
除此之外,磷酸吡哆醛还在血红素合成中的缩合反应、多巴转化为多巴胺以及兴奋性递质谷氨酸转化为抑制性递质γ-氨基丁酸等过程中发挥作用。在赖氨酸分解代谢中,它不参与转氨反应。
生理功能
作为维生素B6的辅酶形式,磷酸吡哆醛在催化反应中发挥关键作用。例如,在氨基酸代谢中的转氨、脱羧或消旋化过程中,磷酸吡哆醛分子内的醛基与α-氨基酸氨基结合形成希夫碱,再通过不同酶蛋白的作用使氨基酸发生相应变化。
应用
磷酸吡哆醛不仅是氨基酸代谢中转氨酶及脱羧酶的辅酶,还能促进谷氨酸脱羧生成γ-氨基丁酸。在临床治疗方面,它被用于提高体内多巴胺含量以缓解帕金森综合症症状等。
化学性质
白色结晶性粉末,无气味,在碱性溶液中呈亮黄色。水溶液需避光低温保存,见光会分解。易溶于甲酸、含水吡啶和稀碱液;微溶于水、乙醇、丙酮、氯仿及乙醚等有机溶剂。
用途
磷酸吡哆醛用于生化研究、孕甾酮特性的结构修饰以及作为酶抑制剂。此外,它还是维生素B6的主要药物之一,适用于治疗急慢性湿疹、皮肤炎等多种皮肤病症状,并且被认为是无毒的。
生产方法
以吡哆醇为原料制备磷酸吡哆醛。具体步骤是将吡哆胺二盐酸盐与无水磷酸反应成吡哆胺磷酸酯,然后通过二氧化锰氧化得到目标产物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 吡哆醛 pyridoxal 66-72-8 C8H9NO3 167.164 吡哆醇磷酸盐 5-hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridylmethyl dihydrogen phosphate 447-05-2 C8H12NO6P 249.16 吡哆胺 5'-磷酸酯 pyridoxamine-5'-phosphate 529-96-4 C8H13N2O5P 248.175 —— pyridoxalphosphate 1499-44-1 C10H13N2O7P 304.196 吡哆醛磷酸酯 gamma-氨基丁酸 4-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(E)-ylidene]-amino}-butyric acid 63221-68-1 C12H17N2O7P 332.25 —— N-(3-hydroxy-2-methyl-5-phosphonooxymethyl-[4]pyridylmethylen)-alanine 98969-65-4 C11H15N2O7P 318.223 2-甲基-3-羟基-4,5-二羟甲基吡啶 5-hydroxy-6-methyl-3,4-pyridinedimethanol 65-23-6 C8H11NO3 169.18 —— Pyridoxal 5-phosphate glutamate 21888-14-2 C13H17N2O9P 376.26 —— (2S,3S)-2-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(Z)-ylidene]-amino}-3-methyl-pentanoic acid 80995-96-6 C14H21N2O7P 360.304 —— pyridoxal benzoyl hydrazone 72343-06-7 C15H15N3O3 285.302 —— (S)-2-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(E)-ylidene]-amino}-3-(1H-indol-3-yl)-propionic acid —— C19H20N3O7P 433.357 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丁-1,3-二烯1,2-二乙烯基苯苯乙烯 4-pyridoxic acid 5'-phosphate 954-27-8 C8H10NO7P 263.144 吡哆醛 pyridoxal 66-72-8 C8H9NO3 167.164 吡哆醇磷酸盐 5-hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridylmethyl dihydrogen phosphate 447-05-2 C8H12NO6P 249.16 吡哆胺 5'-磷酸酯 pyridoxamine-5'-phosphate 529-96-4 C8H13N2O5P 248.175 —— pyridoxal-5'-phosphate hydrate 91239-59-7 C8H12NO7P 265.16 —— pyridoxal phosphate oxime 634-25-3 C8H11N2O6P 262.159 —— 4-<<<3-hydroxy-2-methyl-5-<(phosphonooxy)methyl>-4-pyridynyl>methyl>amino>butanoic acid 22342-63-8 C12H19N2O7P 334.266 —— pyridoxalphosphate 1499-44-1 C10H13N2O7P 304.196 吡哆醛磷酸酯 gamma-氨基丁酸 4-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(E)-ylidene]-amino}-butyric acid 63221-68-1 C12H17N2O7P 332.25 —— N-(3-hydroxy-2-methyl-5-phosphonooxymethyl-[4]pyridylmethylen)-alanine 98969-65-4 C11H15N2O7P 318.223 —— ε-(5-phosphopyridoxinyl)-L-lysine —— C14H24N3O7P 377.334 4-吡哆酸内酯 7-hydroxy-6-methylfuro[3,4-c]pyridin-1(3H)-one 4753-19-9 C8H7NO3 165.148 —— (S)-2-Amino-6-{[1-(3-hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(Z)-ylidene]-amino}-hexanoic acid 2440-59-7 C14H22N3O7P 375.318 3-羧基苯基吡哆胺 5-磷酸酯 3-<<<3-hydroxy-2-methyl-5-<(phosphonooxy)methyl>pyridyl>methyl>amino>benzoic acid 63844-77-9 C15H17N2O7P 368.283 —— (S)-2-Amino-4-{4-[(3-hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-ylmethyl)-amino]-phenyl}-butyric acid —— C18H24N3O7P 425.378 —— 2-Methyl-3-hydroxy-4-cyano-5-hydroxymethyl-pyridin 3990-65-6 C8H8N2O2 164.164 —— Pyridoxal 5-phosphate glutamate 21888-14-2 C13H17N2O9P 376.26 —— (2S,3S)-2-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(Z)-ylidene]-amino}-3-methyl-pentanoic acid 80995-96-6 C14H21N2O7P 360.304 —— 3-(N-[5'-phospho]-pyridoxyl amino)-1-hydroxy-propyliden-1,1-bisphosphonic acid —— C11H21N2O12P3 466.215 —— phosphoric acid mono-(4,5-dihydroxy-6-methyl-[3]pyridylmethyl ester) 5913-68-8 C7H10NO6P 235.133 5'-脱氧吡哆辛 5'-Deoxypyridoxin 4811-03-4 C8H11NO2 153.181 —— 4-(8-methyl-5-phosphonooxy)methyl-3,4-(dihydropyrido<4,3-e>-1,3-oxazin-3-yl)butanoic acid 136027-78-6 C13H19N2O7P 346.277 —— (Z)-5'-O-phosphono-pyridoxylidenerhodanine —— C11H11N2O6PS2 362.324 —— (E)-5'-O-phosphono-pyridoxylidenerhodanine —— C11H11N2O6PS2 362.324 吡哆醛半缩醛 pyridoxal 17281-92-4 C8H9NO3 167.164 —— 2-[[3-hydroxy-2-methyl-5-(phosphonooxymethyl)pyridin-4-yl]methylideneamino]-3-(1H-indol-3-yl)propanoic acid 35314-38-6 C19H20N3O7P 433.357 —— (S)-2-{[1-(3-Hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl)-meth-(E)-ylidene]-amino}-3-(1H-indol-3-yl)-propionic acid —— C19H20N3O7P 433.357 —— N2,S3-(P-Pyridoxyliden)-cystein 899-28-5 C11H15N2O7PS 350.289 - 1
- 2
- 3
反应信息
-
作为反应物:描述:磷酸吡哆醛 在 sodium hydroxide 、 sodium tetrahydroborate 作用下, 生成 1,2-bis-(3-hydroxy-2-methyl-5-phosphonooximethyl-[4]pyridyl)-ethane-1,2-diol参考文献:名称:45.磷酸吡ido醛的光解摘要:DOI:10.1039/jr9580000211
-
作为产物:参考文献:名称:一种磷酸吡哆醛的合成方法摘要:本发明公开了一种合成磷酸吡哆醛(5’‑磷酸吡哆醛)的方法。该方法以盐酸吡哆辛为起始物,通过温和的反应条件氧化得到吡哆醛酸式盐,然后通过磷酸酯化试剂实行磷酸酯化反应,得到磷酸吡哆醛。本发明具有原料易得,路线简单,毒性小,副反应少,产物易分离,易于表征,产率高等特点。公开号:CN108976259A
-
作为试剂:描述:尿苷5'-(2-乙酰氨基-2-脱氧-ALPHA-D-葡糖基焦磷酸酯) 在 磷酸吡哆醛 、 TCCAGGGACCAGCAATGAACTTTCTGATCCTGATTAACAGCGCGCCGAACTACAAGTATTTCTTTTATGAACTGGCGAAAGAAATCGAGAGCCGTGGCCACAACATTTATTTCGCGATCGATAGCCACGTAGCAAGTACCTGGAACCGCTGCCGGAGCTGGACAACAACCAGAACAGCTTCTTTTTCGATAGCTACCTGGAAAAGAACTTTGACAAAAACCTGAGCGTTAGCCACAACAACAACCAAGAGTATTGGGGTGATTACTTCTATAGCGACTACGATCGTTTTCTGACCCACGATTTCAACCTGAACAAGGACAAAAACTACTGGCTGAACGTGAAAGTTAGCCTGGACAGCTTTTTCGAAGACATCATTAAGGATAAACAGATCGACTTTGTGCTGTATGAGAACATTAGCAACAGCTTCGCGTACGCGGCGTATCTGCAATGCACCAAACTGGGCAAGAAATACATCGGTCTGATGGGCAGCCGTCTGCCGAACCACTTTGAAATTCAGAACAGCATCGTTGAGGAAGAGCTGAAGAAACTGGAGATCCTGGCGCAGAAGCCGATTACCCAAGATGAAATGGAGTGGTTCGAAAACTATAAGAAAAGCATCGTGGATATTCAGCCGGACTACATGAAACAAAACGGTCTGGACAACGTGGCGATCAGCCGTATTGTTAAGCTGAACAAATTTCTGAAGGCGCTGCGTCTGCTGACCATCGGCTTCAAGTACAAGCACTACTACGATTACCAGTTCGGTAACCCGTTCATGGTGCCGATCAAGGCGATTCGTGTTAACATTAAACGTTATCTGAACACCAAGAAAAGCCAAAAGTTTTACATCAACAACGACGAACTGGAGATTTGCAGCAGCAAAGAACGTTTTTACATCTATCCGATTCACTTCCATCCGGAGAGCGCACCAGCGTTCTGGCGCCGGAATACACCAACGAGTATAGCAACATCATTAACATCGCGAACAACCTGCC 、 sodium phosphate 、 辅酶 A 、 sodium chloride 、 magnesium chloride 作用下, 反应 81.0h, 生成参考文献:名称:The Retaining Pse5Ac7Ac Pseudaminyltransferase KpsS1 Defines a Previously Unreported glycosyltransferase family (GT118)摘要:
Abstract Cell surface sugar 5,7‐diacetyl pseudaminic acid (Pse5Ac7Ac) is a bacterial analogue of the ubiquitous sialic acid, Neu5Ac, and contributes to the virulence of a number of multidrug resistant bacteria, including ESKAPE pathogens
Pseudomonas aeruginosa , andAcinetobacter baumannii . Despite its discovery in the surface glycans of bacteria over thirty years ago, to date no glycosyltransferase enzymes (GTs) dedicated to the synthesis of a pseudaminic acid glycosidic linkage have been unequivocally characterised in vitro. Herein we demonstrate thatA. baumannii KpsS1 is a dedicated pseudaminyltransferase enzyme (PseT) which constructs a Pse5Ac7Ac‐α(2,6)‐Glcp linkage, and proceeds with retention of anomeric configuration. We utilise this PseT activity in tandem with the biosynthetic enzymes required for CMP‐Pse5Ac7Ac assembly, in a two‐pot, seven enzyme synthesis of an α‐linked Pse5Ac7Ac glycoside. Due to its unique activity and protein sequence, we also assign KpsS1 as the prototypical member of a previously unreported GT family (GT118).DOI:10.1002/anie.202318523
文献信息
-
[EN] ROR-GAMMA INHIBITORS<br/>[FR] INHIBITEURS DE ROR-GAMMA申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2019063748A1公开(公告)日:2019-04-04The present invention relates to compounds of formula I and pharmaceutical compositions comprising compounds of formula I. Compounds of Formula I are useful in treatment of inflammatory, metabolic or autoimmune diseases which are mediated by RORy.本发明涉及公式I的化合物和包含公式I化合物的药物组合物。公式I的化合物在治疗由RORγ介导的炎症性、代谢性或自身免疫性疾病方面是有用的。
-
[EN] NAPHTHALENE CARBOXAMIDE M1 RECEPTOR POSITIVE ALLOSTERIC MODULATORS<br/>[FR] COMPOSÉS DE NAPHTHALÈNE CARBOXAMIDE, MODULATEURS ALLOSTÉRIQUES POSITIFS DU RÉCEPTEUR M1申请人:MERCK SHARP & DOHME公开号:WO2011149801A1公开(公告)日:2011-12-01The present invention is directed to naphthalene carboxamide compounds of formula (I) which are M1 receptor positive allosteric modulators and that are useful in the treatment of diseases in which the M1 receptor is involved, such as Alzheimers disease, schizophrenia, pain or sleep disorders. The invention is also directed to pharmaceutical compositions comprising the compounds and to the use of the compounds and compositions in the treatment of diseases mediated by the M1 receptor.本发明涉及式(I)的萘甲酰胺化合物,它们是M1受体阳性变构调节剂,可用于治疗M1受体参与的疾病,如阿尔茨海默病、精神分裂症、疼痛或睡眠障碍。该发明还涉及包含这些化合物的药物组合物,以及在治疗由M1受体介导的疾病中使用这些化合物和组合物。
-
[EN] PROTEIN TYROSINE PHOSPHATASE INHIBITORS AND METHODS OF USE THEREOF<br/>[FR] INHIBITEURS DE PROTÉINE TYROSINE PHOSPHATASE ET LEURS PROCÉDÉS D'UTILISATION申请人:CALICO LIFE SCIENCES LLC公开号:WO2020186199A1公开(公告)日:2020-09-17Provided herein are compounds, compositions, and methods useful for inhibiting protein tyrosine phosphatase, e.g., protein tyrosine phosphatase non-receptor type 2 (PTPN2) and/or protein tyrosine phosphatase non-receptor type 1 (PTPN1), and for treating related diseases, disorders and conditions favorably responsive to PTPN 1 or PTPN2 inhibitor treatment, e.g., a cancer or a metabolic disease.
-
[EN] PROCESS FOR THE SYNTHESIS OF RAMELTEON AND ITS INTERMEDIATES<br/>[FR] PROCÉDÉ DE SYNTHÈSE DU RAMELTEON ET DE SES INTERMÉDIAIRES申请人:TEVA PHARMA公开号:WO2010045565A1公开(公告)日:2010-04-22The present invention provides processes and intermediates for the synthesis of ramelteon.本发明提供了拉莫特普合成的工艺和中间体。
-
Novel Bicyclic Pyridinones申请人:Pettersson Martin Youngjin公开号:US20120252758A1公开(公告)日:2012-10-04Compounds and pharmaceutically acceptable salts of the compounds are disclosed, wherein the compounds have the structure of Formula I as defined herein. Corresponding pharmaceutical compositions, methods of treatment, methods of synthesis, and intermediates are also disclosed.所述化合物及其药用可接受的盐被披露,其中所述化合物具有如本文所定义的Formula I的结构。相应的药物组合物、治疗方法、合成方法和中间体也被披露。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
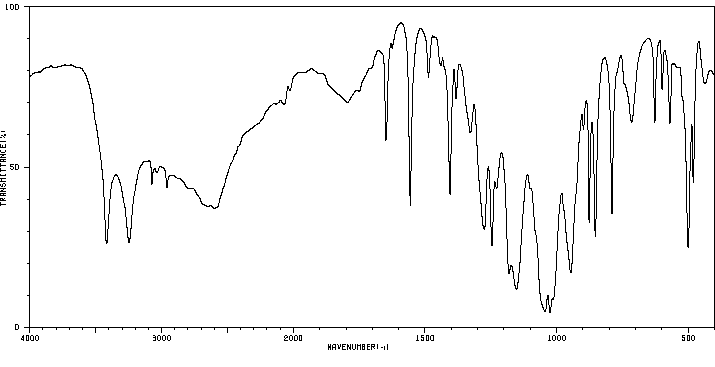
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息