sodium hydrogen L-tartrate | 526-94-3
中文名称
——
中文别名
——
英文名称
sodium hydrogen L-tartrate
英文别名
(R,R)-tartaric acid monosodium salt;mono-sodium L-tartrate;sodium hydrogen tartrate;[Na(μ6-HTar)]n;Lg-tartaric acid ; sodium hydrogen-Lg-tartrate;Lg-Weinsaeure; Natrium-hydrogen-Lg-tartrat;sodium acid tartrate;Sodium bitartrate;sodium;(2R,3R)-2,3,4-trihydroxy-4-oxobutanoate
CAS
526-94-3
化学式
C4H5O6*Na
mdl
——
分子量
172.07
InChiKey
NKAAEMMYHLFEFN-ZVGUSBNCSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:253 °C (dec.)(lit.)
-
溶解度:H2O: 0.1 M , 20 °C, 澄清,无色
-
LogP:-1.426 (est)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-6.45
-
重原子数:11
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:118
-
氢给体数:3
-
氢受体数:6
安全信息
-
TSCA:Yes
-
安全说明:S22,S24/25
-
WGK Germany:2
-
海关编码:2918199090
-
RTECS号:WW8225000
-
储存条件:1. 储存在阴凉、干燥、通风良好的仓库中。 2. 远离火源和热源,避免阳光直射,确保包装密封。 3. 与酸类和食用化学品分开存放,禁止混存。储区应备有合适的材料以处理泄漏物。
SDS
| Name: | Sodium Hydrogen Tartrate Monohydrate Material Safety Data Sheet |
| Synonym: | Butanedioic acid, 2,3-dihydroxy- (2R,3R)-, monosodium salt, monohydrate; Sodium hydrogen tartrate monohydrate |
| CAS: | 526-94-3 |
Synonym:Butanedioic acid, 2,3-dihydroxy- (2R,3R)-, monosodium salt, monohydrate; Sodium hydrogen tartrate monohydrate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 526-94-3 | Sodium bitartrate monohydrate | 100 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation.
Chronic:
May cause kidney damage.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Dusts may be combustible when exposed to heat, flame, or oxidizing agents.
Extinguishing Media:
For small fires, use water spray, dry chemical, carbon dioxide or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid breathing dust.
Storage:
Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 526-94-3: CAS# 6131-98-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: none reported
pH: acidic in solution
Vapor Pressure: Not applicable.
Viscosity: Not applicable.
Boiling Point: Not applicable.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: 426 deg F
Solubility in water: Soluble.
Specific Gravity/Density: Not available.
Molecular Formula: C4H6O6.Na.H2O
Molecular Weight: 190.09
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
High temperatures, dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 526-94-3: WW8225000 CAS# 6131-98-2 unlisted.
LD50/LC50:
CAS# 526-94-3: Oral, mouse: LD50 = 4360 mg/kg.
CAS# 6131-98-2.
Carcinogenicity:
Sodium hydrogen tartrate, anhydrous - Not listed by ACGIH, IARC, or NTP.
Sodium bitartrate monohydrate - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 526-94-3: 1
CAS# 6131-98-2: No information available.
Canada
CAS# 526-94-3 is listed on Canada's DSL List.
CAS# 526-94-3 is not listed on Canada's Ingredient Disclosure List.
CAS# 6131-98-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 526-94-3 is listed on the TSCA inventory.
CAS# 6131-98-2 is not on the TSCA Inventory because it is a hydrate.
It is considered to be listed if the CAS number for the anhydrous form
is on the inventory (40CFR720.3(u)(2)).
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
酒石酸氢钠单水合物可溶于9倍的冷水和1.8倍的热水中,不溶于乙醇。加热至100℃时脱水变成无水物,在干燥空气中于234℃分解。DL-型为无色单斜或三斜系晶体,易溶于沸水且不溶于乙醇。在100℃时脱水变为无水物,并在219℃时分解。
应用酒石酸氢钠单水合物主要用于医药化工领域,例如制备锑掺杂二氧化锡纳米粉体。
制备将等摩尔量的酒石酸和氢氧化钠混合物水溶液冷却,或向酒石酸钠水溶液中加入等摩尔的酒石酸后冷却。必要时可加入乙醇以获得酒石酸氢钠单水合物。
性状白色晶体。
性质酒石酸氢钠为无色透明结晶或白色结晶性粉末,密度1.818,在150℃时失去2个结晶水。易溶于水且水溶液呈弱碱性,不溶于乙醇。
用途作为螯合剂、酸味调整剂、缓冲剂、乳化盐以及抗氧化剂和抗氧化增效剂使用。也可用作分析试剂,并用于培养基的制备及生化试剂中,如检验钾和制备培养基等。
反应信息
-
作为反应物:参考文献:名称:WO2008/131149摘要:公开号:
-
作为产物:参考文献:名称:钠和银纳米配位聚合物的固-固和固-液转化摘要:摘要通过声化学方法合成的三维钠配位聚合物[Na(μ6-HTar)] n(1),(H2Tar =(+)-酒石酸)固态转化为二维银配位在1与AgNO3进行机械化学阳离子交换反应时,观察到聚合物[Ag(μ3-HTar)(H2O)] n(2)。还已经研究了通过固态反应将2转化为1的方法。这些样品通过红外光谱,X射线粉末衍射(XRD),热重分析和差热分析(TG-DTA),能量色散X射线光谱(EDAX)和扫描电子显微镜(SEM)进行表征。观察到化合物1向化合物2的转化是不可逆的。此外,还证实了[Ag(μ3-HTar)(H2O)] n(2)在水中固液反应过程中转化为[Ag2(HTar)] n(3)。DOI:10.1016/j.poly.2019.03.032
-
作为试剂:描述:乙酰乙酸甲酯 在 镍 sodium hydroxide 、 氢气 、 sodium hydrogen L-tartrate 作用下, 以 四氢呋喃 为溶剂, 373.0 ℃ 、10.0 MPa 条件下, 生成 (S)-3-羟基丁酸甲酯 、 (R)-3-羟基丁酸甲酯参考文献:名称:In situ Chiral Modification of Nickel Catalysts for Enantioselective Hydrogenation of Methyl Acetoacetate摘要:研究了SiO2支持和Raney镍催化剂的手性修饰。这些催化剂用于甲基乙酰丙酮(MAA)的不对称氢化反应,制备(
R )-和(S )-对映体的甲基3-羟基丁酸酯。讨论了修饰参数(例如修饰剂的类型和浓度、共修饰剂和其他添加剂的存在、修饰溶液的pH值)对MAA氢化的对映选择性的影响。还评估了Ni/SiO2的in situ 修饰的特征,并将所得结果与传统(预修饰)方法进行了比较。在一系列实验中,优化了两种催化剂的传统和in situ 方法的参数。发现in situ 修饰的Ni/SiO2由于选择性和活性几乎没有下降,因此特别适合重复使用。DOI:10.1135/cccc20051642
文献信息
-
DE469554申请人:——公开号:——公开(公告)日:——
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: K: MVol.6, 2.22, page 1227 - 1230作者:DOI:——日期:——
表征谱图
-
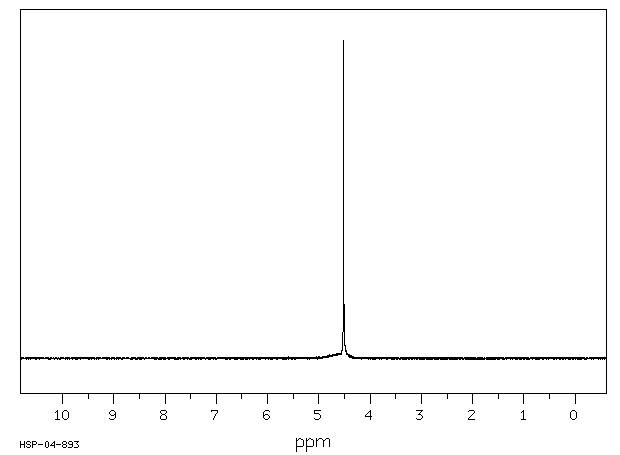
氢谱1HNMR
-
质谱MS
-
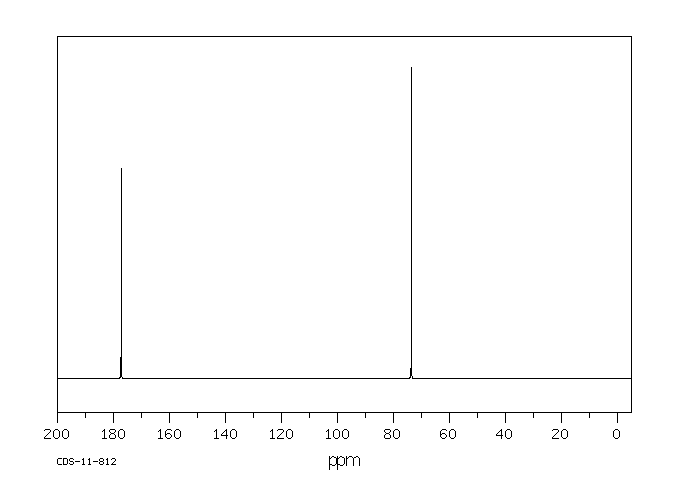
碳谱13CNMR
-
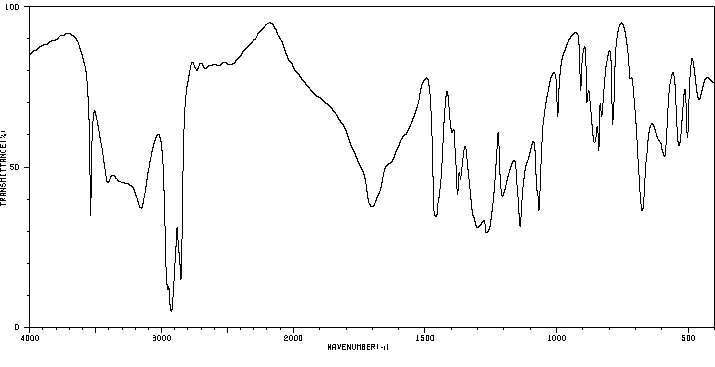
红外IR
-
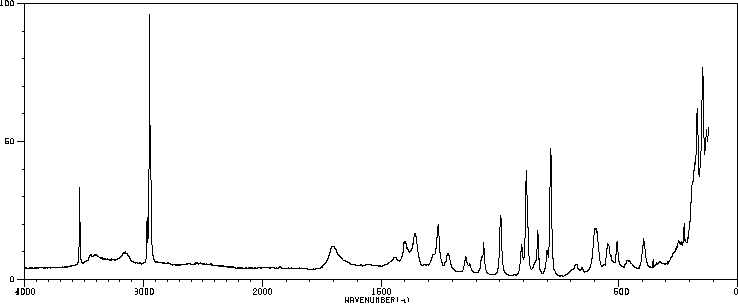
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷