5-氯-2-甲氧基苯磺酰氯 | 22952-32-5
中文名称
5-氯-2-甲氧基苯磺酰氯
中文别名
五氯二甲基苯磺酰氯化物;5-氯-2-甲氧基基苯磺酰氯
英文名称
5-chloro-2-methoxybenzenesulfonyl chloride
英文别名
——
CAS
22952-32-5
化学式
C7H6Cl2O3S
mdl
MFCD00052959
分子量
241.095
InChiKey
FCJGLIMDVOTBLO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100-104 °C
-
沸点:344.6±32.0 °C(Predicted)
-
密度:1.487±0.06 g/cm3(Predicted)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45,S8
-
危险类别码:R34
-
海关编码:2909309090
-
危险品运输编号:3261
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:保持贮藏器密封,并将其放入一个紧密的容器中。存放在阴凉、干燥的地方。
SDS
| Name: | 5-Chloro-2-methoxybenzenesulfonyl chloride Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 22952-32-5 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 22952-32-5 | 5-Chloro-2-methoxybenzenesulfonyl chlo | 100 | unlisted |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam. Do NOT get water inside containers.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing.
Keep container tightly closed. Do not ingest or inhale. Do not allow contact with water. Use only in a chemical fume hood. Discard contaminated shoes. Keep from contact with moist air and steam.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Corrosives area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 22952-32-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 101 -103 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
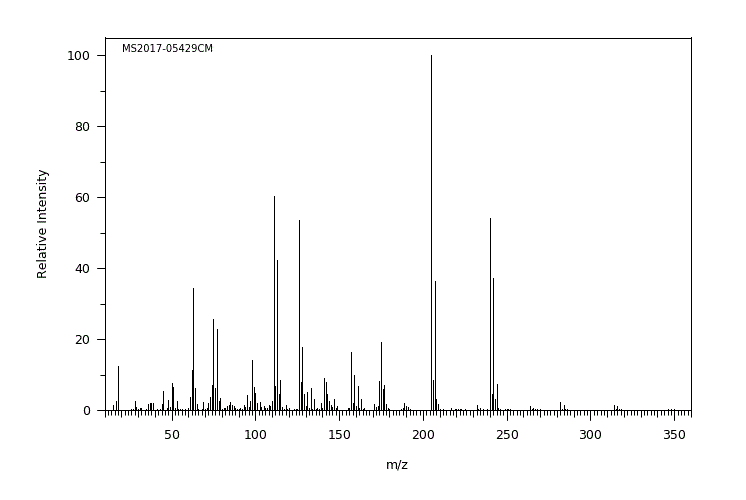
Molecular Formula: C7H6Cl2O3S
Molecular Weight: 241.0062
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Dust generation, exposure to moist air or water.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, oxides of sulfur, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 22952-32-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Chloro-2-methoxybenzenesulfonyl chloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: Pseudomonas putida:
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: III
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 22952-32-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 22952-32-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 22952-32-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
合成制备方法
目前暂无详细描述。
用途目前暂无详细描述。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯苯甲醚-2-磺酸 4-chloroanisole-2-sulfonic acid 46170-99-4 C7H7ClO4S 222.649 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-chloro-2-methoxybenzenesulfonamide 82020-51-7 C7H8ClNO3S 221.664 —— 5-chloro-2-methoxy-N-methylbenzenesulfonamide 364067-04-9 C8H10ClNO3S 235.691 —— 5-chloro-2-methoxybenzenesulfonohydrazide 22952-33-6 C7H9ClN2O3S 236.679 —— 5-chloro-2-methoxy-N-propylbenzenesulfonamide —— C10H14ClNO3S 263.745
反应信息
-
作为反应物:描述:5-氯-2-甲氧基苯磺酰氯 在 ammonium hydroxide 作用下, 反应 19.0h, 以100%的产率得到5-chloro-2-methoxybenzenesulfonamide参考文献:名称:[EN] PYRIDO-OXAZINONE DERIVATIVES AS TNAP INHIBITORS
[FR] DÉRIVÉS DE PYRIDO-OXAZINONE UTILISÉ COMME INHIBITEURS DE TNAP摘要:本发明涉及一种具有出色的组织非特异性碱性磷酸酶抑制活性的化合物或其药理学可接受的盐。本发明提供一种由以下式(I)表示的化合物:其中R1代表氢原子、可选择取代的Cl-6烷基基团等,R2和R3相同或不同,各自代表氢原子、可选择取代的Cl-6烷基基团等,R4和R5相同或不同,各自代表氢原子、可选择取代的Cl-6烷基基团等,R6代表氢原子或等,每个R7可能相同或不同,每个可以代表可选择取代的Cl-6烷氧基团等,X代表-CH=,-C(-R7)=,或-N=,m代表1到4,或其药理学可接受的盐。公开号:WO2017007943A1 -
作为产物:参考文献:名称:构建特权二苯并[ b,f ] [1,4,5]奥沙西平5,5-二氧化物及其杂环等排物的新颖灵活策略摘要:仲邻羟基苯磺酰胺已作为一种双亲电子伙伴被研究出来,它是一种实用,简单,经济的二苯并[ b,f ] [1,4,5]氧杂氮杂卓-5,5-二氧化物及其杂环类似物的方法。用于药物设计的特权三环支架的未开发版本。该反应在常规加热条件下平稳且区域特异性地进行,并以良好至优异的产率递送目标化合物。该方法代表了二苯并[ b,f] [1,4,5] oxathiazepine-5,5-dioxide支架,可以根据取代方式使用新的化学空间。特别地,可以通过在环化反应中改变双亲电子芳族配偶的性质,方便地在三环框架内改变杂环。这已经通过合成三个迄今未描述的杂环三环支架得到了证明。如我们对一系列类似的成环过程所观察到的那样,环化反应是通过Smiles重排进行的。DOI:10.1016/j.tet.2016.10.008
-
作为试剂:描述:resin-bound 2-Amino-4,7-dihydro-5H-thieno[2,3-c]pyridine-3,6-dicarboxylic acid 6-tert-butyl ester 在 烟酰氯 、 丙酰氯 、 N,N-二异丙基乙胺 、 异丁酰氯 、 4-正丁氧基苯甲酰氯 、 7-甲氧基苯并呋喃-2-甲酸 、 4-氟苯磺酰氯 、 4-乙酰基苯磺酰氯 、 1-萘磺酰氯 、 5-氯-2-甲氧基苯磺酰氯 、 对乙酰氨基苯甲酸 、 1-羟基苯并三唑 、 5-氯-1,3-二甲基-1H-吡唑-4-磺酰氯 、 N,N'-二异丙基碳二亚胺 、 2,4-二氟苯甲酸 作用下, 以 二氯甲烷 、 三氟乙酸 、 四氢呋喃 、 N,N-di-isopropylcarbodiimide 为溶剂, 反应 96.5h, 生成 6-(4-Fluoro-benzenesulfonyl)-2-isobutyrylamino-4,5,6,7-tetrahydro-thieno[2,3-c]pyridine-3-carboxylic acid 、 6-(4-Acetylamino-benzoyl)-2-isobutyrylamino-4,5,6,7-tetrahydro-thieno[2,3-c]pyridine-3-carboxylic acid参考文献:名称:Bicyclic thiophene derivatives and combinatorial libraries thereof摘要:本发明涉及以下式子的新型双环硫吡啶衍生物化合物:1其中R1至R2、X和n具有本文所提供的含义。本发明还涉及包含两个或更多这样的化合物的组合式库,以及制备双环硫吡啶衍生物化合物的方法。公开号:US20030232994A1
文献信息
-
[EN] ARYL-1,3-AZOLE DERIVATIVES AND METHODS FOR INHIBITING HEPARNASE ACTIVITY<br/>[FR] DERIVES D'ARYL-1,3-AZOLE ET PROCEDES PERMETTANT D'INHIBER L'ACTIVITE DE L'HEPARANASE申请人:IMCLONE SYSTEMS INC公开号:WO2005030206A1公开(公告)日:2005-04-07The present invention encompasses heparanase inhibitors, particularly to certain 2-substituted heteroaryl-fused and aryl-fused carbazole derivatives that inhibit heparanase, pharmaceutical compositions that contain the compounds, methods for making the compounds, and methods of treating heparanase-dependent diseases and conditions in mammals by administering a therapeutically effective amount of the compounds to the mammals.
-
[EN] SULFONAMIDE COMPOUNDS HAVING TNAP INHIBITORY ACTIVITY<br/>[FR] COMPOSÉS DE SULFONAMIDE AYANT UNE ACTIVITÉ INHIBITRICE DE TNAP申请人:DAIICHI SANKYO CO LTD公开号:WO2018119444A1公开(公告)日:2018-06-28The present invention relates to a compound or a pharmacologically acceptable salt thereof having excellent tissue non-specific alkaline phosphatase inhibitory activity. The present invention provides a compound represented by the formula (I) or a pharmacologically acceptable salt thereof.本发明涉及一种具有优异的组织非特异性碱性磷酸酶抑制活性的化合物或其药理学上可接受的盐。本发明提供一种由式(I)表示的化合物或其药理学上可接受的盐。
-
Synthesis, in vitro α-amylase inhibitory, and radicals (DPPH & ABTS) scavenging potentials of new N-sulfonohydrazide substituted indazoles作者:Rafaila Rafique、Khalid Mohammed Khan、Arshia、Sridevi Chigurupati、Abdul Wadood、Ashfaq Ur Rehman、Uzma Salar、Vijayan Venugopal、Shahbaz Shamim、Muhammad Taha、Shahnaz PerveenDOI:10.1016/j.bioorg.2019.103410日期:2020.1used to treat type-II diabetes mellitus, but also linked to several harmful effects. Therefore, it is essential to explore new and nontoxic antidiabetic agents with additional antioxidant properties. In this connection, a series of new N-sulfonohydrazide substituted indazoles 1-19 were synthesized by multistep reaction scheme and assessed for in vitro α-amylase inhibitory and radical (DPPH and ABTS)α-淀粉酶的过度表达会引起高血糖症,从而导致许多生理并发症,包括氧化应激,这是糖尿病最常见的问题之一。市售的α-淀粉酶抑制剂,例如阿卡波糖,伏格列波糖和米格列醇,用于治疗II型糖尿病,但也与多种有害作用有关。因此,必须探索具有其他抗氧化特性的新型无毒抗糖尿病药。在这方面,通过多步反应方案合成了一系列新的N-磺酰肼取代的吲唑1-19,并评估了其体外α-淀粉酶抑制和自由基(DPPH和ABTS)清除性能。所有化合物均通过不同的光谱技术进行了全面表征,包括1H,13C NMR,EI-MS,HREI-MS,ESI-MS和HRESI-MS。与标准阿卡波糖(IC50 1.20±0.09 µM)相比,化合物显示出有希望的α-淀粉酶抑制活性(IC50 = 1.23±0.06-4.5±0.03 µM)。此外,与具有清除活性的标准抗坏血酸相比,所有衍生物均被发现具有良好至中度的DPPH(IC50 2.01±0.13-5
-
[EN] SULFONAMIDE COMPOUNDS AND USES AS TNAP INHIBITORS<br/>[FR] COMPOSÉS SULFONAMIDES ET LEURS UTILISATIONS EN TANT QU'INHIBITEURS DE TNAP申请人:SANFORD BURNHAM MED RES INST公开号:WO2013126608A1公开(公告)日:2013-08-29Described herein are compounds that modulate the activity of TNAP. In some embodiments, the compounds described herein inhibit TNAP. In certain embodiments, the compounds described herein are useful in the treatment of conditions associated with hyper- mineralization.本发明描述了调节TNAP活性的化合物。在某些实施例中,本发明描述的化合物抑制TNAP。在某些实施例中,本发明描述的化合物可用于治疗与过度矿化相关的病症。
-
Synthesis of 1-aroyl(1-arylsulfonyl)-4-bis(trifluoromethyl)alkyl semicarbazides as potential physiologically active compounds作者:Elena L. Luzina、Anatoliy V. PopovDOI:10.1016/j.jfluchem.2013.01.033日期:2013.41,1-Bis(trifluoromethyl)alkyl isocyanates obtained from perfluoroisobutene (PFIB) react with aroyl(arylsulfonyl)hydrazines. Twenty eight prospective biologically active polyfluorinated 1,4-substituted semicarbazides were synthesized. The structure of each new product was confirmed by analytical and spectroscopic methods. The Lipinski's and Gelovani's parameters were then calculated. Two adjustments
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
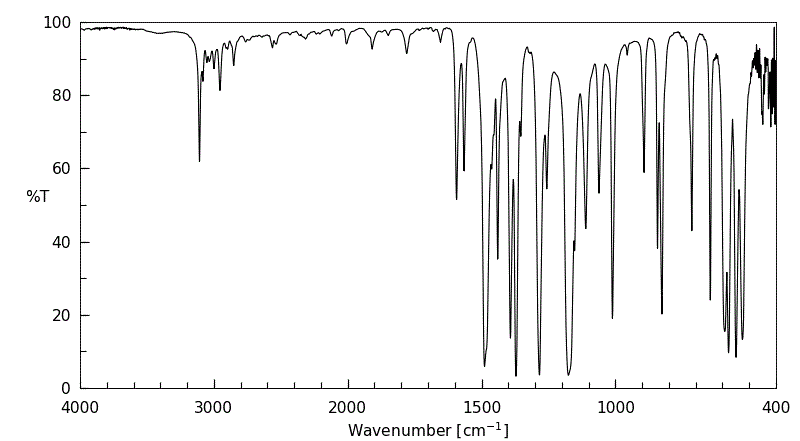
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫