苯基-N-乙酰基-beta-D-氨基葡糖苷 | 5574-80-1
中文名称
苯基-N-乙酰基-beta-D-氨基葡糖苷
中文别名
苯基-2-乙酰氨基-2-脱氧-Β-D-葡萄糖苷
英文名称
phenyl 2-acetamido-2-deoxy-β-D-glucopyranoside
英文别名
Phenyl 2-acetamido-2-deoxy-beta-D-glucopyranoside;N-[(2S,3R,4R,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-2-phenoxyoxan-3-yl]acetamide
CAS
5574-80-1
化学式
C14H19NO6
mdl
MFCD00067652
分子量
297.308
InChiKey
ZUJDLWWYFIZERS-DHGKCCLASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:21
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:108
-
氢给体数:4
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯基 2-乙酰氨基-3,4,6-三-O-乙酰基-2-脱氧吡喃己糖苷 1-O-phenyl-2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranose 13089-21-9 C20H25NO9 423.42 —— D-GlcNAc 7512-17-6 C8H15NO6 221.21 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phenyl 2-acetamido-2-deoxy-4,6-O-isopropylidene-β-D-glucopyranoside 404947-30-4 C17H23NO6 337.373
反应信息
-
作为反应物:描述:苯基-N-乙酰基-beta-D-氨基葡糖苷 在 吡啶 、 4-二甲氨基吡啶 、 磷酸 、 三乙胺 、 乙酰氯 、 N,N'-二环己基碳二亚胺 作用下, 以 甲醇 、 乙醚 、 二氯甲烷 、 二甲基亚砜 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 79.16h, 生成 diallyl [phenyl 2-acetamido-3-O-benzoyl-2,4-dideoxy-β-L-threo-hex-4-enopyranoside-6-yl]-phosphonate参考文献:名称:Efficient Sialyltransferase Inhibitors Based on Glycosides of N-Acetylglucosamine摘要:D-Glucosamine was transformed into phenyl and 2-benzoyloxyethyl N-acetylglucosamine beta-glycosides 6a and 6b, respectively. Transformation of 6a,b into 6-O-unprotected N-acetylglucosamine derivatives 9a,b permitted the generation of an aldehyde group in the 6-position, Treatment of these intermediates with base afforded unsaturated aldehyde derivatives 10a,b, which are structural mimics of 2,3-dehydroneuraminic acid. H-Phosphonate addition to the aldehyde group and attachment of the cytidine monophosphate residue to the generated hydroxy group gave fully protected transition state analogues of cytidine monophosphate-N-acetylneuraminic acid 14a,b. Liberation of the unprotected compounds 1ah,l and 1lbh,l led to excellent inhibitors of alpha(2-6) -sialyltransferase from rat liver. Variation of the protective group cleavage procedure for 14a,b led to formal loss of phosphate, thus resulting in diene derivatives (E)-/(Z)-2a,b, which also exhibited inhibitory properties.DOI:10.1021/ja017370n
-
作为产物:描述:2-乙酰氨基-3,4,6-三-O-乙酰-2-脱氧-α-D-吡喃葡萄糖酰基氯 在 sodium hydroxide 、 苄基三乙基氯化铵 、 sodium methylate 作用下, 以 甲醇 、 二氯甲烷 为溶剂, 反应 1.0h, 生成 苯基-N-乙酰基-beta-D-氨基葡糖苷参考文献:名称:O-GlcNAcase 在没有一般酸催化的情况下催化硫糖苷的裂解摘要:O-GlcNAcase 使用涉及底物辅助催化和一般酸/碱催化的催化机制,催化从翻译后修饰蛋白质的丝氨酸和苏氨酸残基中去除 N-乙酰氨基葡糖残基。由于硫代糖苷被广泛认为对糖苷酶的水解具有抗性,因此令人惊讶地发现 O-GlcNAcase 还催化 S-糖苷的有效水解。O-GlcNAcase 催化的一系列芳基 S-和 O-糖苷水解的 Brønsted 分析和 pH 活性研究表明,O-GlcNAcase 在没有一般酸催化帮助的情况下影响硫糖苷的水解。O-和S-糖苷的α-氘动力学同位素效应,以及使用N-氟乙酰-β-糖苷的类似塔夫脱的分析,表明 O-GlcNAcase 通过稳定晚期过渡态来完成硫糖苷的水解。对于 S-糖苷,与 O-糖苷相比,这种过渡态显示出更多的来自 2-乙酰胺基团的亲核参与。控制 O-GlcNAcase 催化的 O-和 S-糖苷水解的速率常数与先前为结构相似的 O,O-和 O,S-缩醛DOI:10.1021/ja0567687
文献信息
-
Probing Synergy between Two Catalytic Strategies in the Glycoside Hydrolase <i>O</i>-GlcNAcase Using Multiple Linear Free Energy Relationships作者:Ian R. Greig、Matthew S. Macauley、Ian H. Williams、David J. VocadloDOI:10.1021/ja904506u日期:2009.9.23revealing changes in mechanism, transition state, and rate-determining step upon concomitant variation of both nucleophilic strength and leaving group abilities are observed. The observed changes in mechanism reflect the roles played by the enzymic general acid and the catalytic nucleophile. Significantly, these results illustrate how the enzyme synergistically harnesses both modes of catalysis; a feature that人类 O-GlcNAcase 在调节丝氨酸和苏氨酸残基与 β-O 连接的 N-乙酰氨基葡萄糖单糖单元 (O-GlcNAc) 的翻译后修饰方面发挥着重要作用。O-GlcNAcase 的机制涉及底物的 2-乙酰氨基基团的亲核参与以取代糖苷连接的离去基团。这种酶对底物结构变化的耐受性使我们能够使用几个系列的底物来表征 O-GlcNAcase 过渡态,以产生多个同时的自由能关系。观察到伴随亲核强度和离去基团能力变化的机制、过渡态和速率决定步骤的变化模式。观察到的机制变化反映了酶通酸和催化亲核试剂所起的作用。重要的是,这些结果说明了酶如何协同利用两种催化模式;许多小分子催化模型都没有的特征。这些研究还表明了氧代碳鎓离子中间体在 O-GlcNAcase 催化的氨基葡萄糖水解中的动力学意义,探索了使用酶过渡态结构的非原子研究可能学到的知识的局限性,并提供了关于保留超家族的一般见解糖苷水解酶作为有效的催化剂。
-
Facile Formation of β‐thioGlcNAc Linkages to Thiol‐Containing Sugars, Peptides, and Proteins using a Mutant GH20 Hexosaminidase作者:Gregor Tegl、John Hanson、Hong‐Ming Chen、David H Kwan、Andrés G. Santana、Stephen G. WithersDOI:10.1002/anie.201809928日期:2019.2.4including S-GlcNAcylated proteins, is reported, using a thioglycoligase derived from a GH20 hexosaminidase from Streptomyces plicatus in which the catalytic acid/base glutamate has been mutated to an alanine (SpHex E314A). This robust, easily-prepared, engineered enzyme uses GlcNAc and GalNAc donors and couples them to a remarkably diverse set of thiol acceptors. Thioglycoligation using 3-, 4-, and 6-thiosugar
-
Scope of the DMC mediated glycosylation of unprotected sugars with phenols in aqueous solution作者:Xin Qiu、Antony J. FairbanksDOI:10.1039/d0ob01727b日期:——sugars in aqueous solution using 2-chloro-1,3-dimethylimidazolinium chloride (DMC) and triethylamine in the presence of para-nitrophenol allows direct stereoselective conversion to the corresponding 1,2-trans para-nitrophenyl glycosides without the need for any protecting groups. The reaction is applicable to sulfated and phosphorylated sugars, but not to ketoses or uronic acids or their derivatives
-
4-Deoxy-substrates for β-N-acetylhexosaminidases: How to make use of their loose specificity作者:Kristýna Slámová、Radek Gažák、Pavla Bojarová、Natallia Kulik、Rudiger Ettrich、Helena Pelantová、Petr Sedmera、Vladimír KřenDOI:10.1093/glycob/cwq058日期:2010.8screened against a panel of β-N-acetylhexosaminidases (extracellular and intracellular) from various sources (fungal, human, animal, plant and bacterial) for hydrolysis. The results of this screening are reported here, as well as the structures of three novel 4′-deoxy-disaccharides prepared by transglycosylation reaction with high yields (52% total disaccharide fraction) using β-N-acetylhexosaminidaseβ- N-乙酰己糖胺酶具有所谓的摆动特异性,这意味着它们可以以葡萄糖和半乳糖的形式裂解底物,其活性比取决于酶的来源。在这里,我们提出了一个新发现,即真菌β- N-乙酰基己糖胺酶能够高产率地水解和转移4-脱氧-N-乙酰基己糖胺类。这清楚地表明,在底物吡喃糖环上的4-羟基部分对于底物与酶活性位点的结合不是必需的,这也通过被测化合物分子对接到β- N-乙酰基己糖胺酶活性位点的模型中得到证实。来自米曲霉。合成了一组四个4-脱氧-N-乙酰基己糖胺,并针对来自各种来源(真菌,人,动物,植物和细菌)的一组β- N-乙酰基己糖苷酶(细胞外和细胞内)进行了水解筛选。此处报道了该筛选的结果,以及三种新的4'-脱氧二糖的结构,这些结构是通过使用来自Talaromyces flavus的β- N-乙酰基己糖胺酶以高收率(占总二糖部分的52%)进行转糖基化反应制得的。
-
Chemical synthesis of 4-azido-β-galactosamine derivatives for inhibitors of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase作者:Seanghai Hor、Takumi Kodama、Nobuo Sugiura、Hikaru Kondou、Mio Yanagida、Keiya Yanagisawa、Aoki Shibasawa、Bunta Tsuzuki、Naoto Fukatsu、Kazuya Nagao、Kenji Yamana、Kazuya I. P. J. Hidari、Hideto Watanabe、Osami Habuchi、Hirofumi NakanoDOI:10.1007/s10719-018-9839-2日期:2018.10family of new 4-acylamino-β-GalNAc derivatives and 4-azido-β-GalNAc derivatives were synthesized for their potential application as inhibitors of GalNAc4S-6ST. The target compounds were evaluated for their inhibitory activities against GalNAc4S-6ST. The results revealed that 4-pivaloylamino- and 4-azido-β-GalNAc derivatives displayed evident activities against GalNAc4S-6ST with IC50 value ranging from 0硫酸软骨素E(CS-E)在从病毒感染到神经再生的各种过程中起着至关重要的作用。N-乙酰半乳糖胺4-硫酸盐6- O生成的区域特异性硫酸盐模式-磺基转移酶(GalNAc4S-6ST)是其生物学活性的主要结构决定因素。GalNAc4S-6ST的抑制剂可以用作了解CS-E的生理功能及其对人类疾病的潜在治疗先导的有力工具。合成了一系列新的4-酰基氨基-β-GalNAc衍生物和4-叠氮基-β-GalNAc衍生物,作为其潜在的GalNAc4S-6ST抑制剂的应用。评价目标化合物对GalNAc4S-6ST的抑制活性。结果显示4-新戊酰氨基-和4-叠氮基-β-GalNAc衍生物表现出明显的针对GalNAc4S-6ST的活性,IC 50值为0.800至0.828mM。它们显示出比用作对照的苄基D-GalNAc4S更高的活性。
表征谱图
-
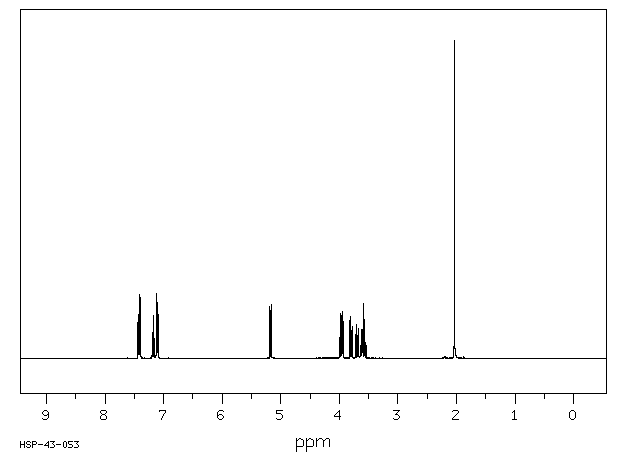
氢谱1HNMR
-
质谱MS
-
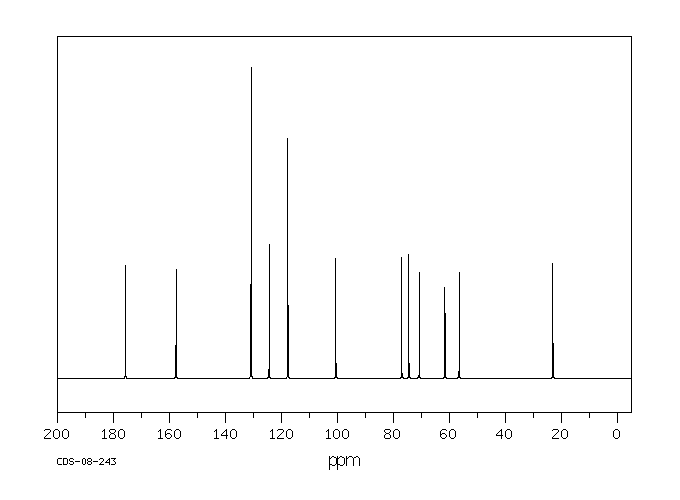
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷