苯甲酸乙基己酯 | 5444-75-7
中文名称
苯甲酸乙基己酯
中文别名
苯甲酸2-乙基己酯;2-乙基己基苯甲酸酯
英文名称
Velate 368
英文别名
benzoic acid 2-ethylhexyl ester;(2-ethyl-1-hexyl) benzoate;2-ethylhexyl monobenzoate;2-ethyl-1-hexyl benzoate;2-ethyl-n-hexyl benzoate;2-ethylhexyl benzoate
CAS
5444-75-7
化学式
C15H22O2
mdl
——
分子量
234.338
InChiKey
UADWUILHKRXHMM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:170 °C / 20mmHg
-
密度:0,97 g/cm3
-
LogP:6.21 at 20℃
-
物理描述:Liquid
-
溶解度:Practically insoluble or insoluble in water
-
折光率:1.487-1.497
-
保留指数:1674
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:17
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.53
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36,S37,S39
-
危险类别码:R36/37/38
-
海关编码:2916310090
-
WGK Germany:3
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温、避光、存放在阴凉干燥处并密封保存。
SDS
苯甲酸2-乙基己酯 修改号码:5
模块 1. 化学品
产品名称: 2-Ethylhexyl Benzoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯甲酸2-乙基己酯
百分比: >99.0%(GC)
CAS编码: 5444-75-7
俗名: Benzoic Acid 2-Ethylhexyl Ester
分子式: C15H22O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
苯甲酸2-乙基己酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 170 °C/2.7kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.97
溶解度:
[水] 无资料
[其他溶剂] 无资料
苯甲酸2-乙基己酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
苯甲酸2-乙基己酯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Ethylhexyl Benzoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯甲酸2-乙基己酯
百分比: >99.0%(GC)
CAS编码: 5444-75-7
俗名: Benzoic Acid 2-Ethylhexyl Ester
分子式: C15H22O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
苯甲酸2-乙基己酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 170 °C/2.7kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.97
溶解度:
[水] 无资料
[其他溶剂] 无资料
苯甲酸2-乙基己酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
苯甲酸2-乙基己酯 修改号码:5
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Method of treating tire surfaces摘要:本申请涉及一种用于处理轮胎的方法,使轮胎在使用时表现更佳,特别是在赛车中使用。本文所披露的方法特别适用于卡丁车赛车,尽管该方法可用于其他应用,其中轮胎的粘附性和硬度在制造后需要改变。该方法还包括清洁步骤和配方。该方法涉及有选择地使用各种酯类来按需改变轮胎特性。公开号:US07837778B1
-
作为产物:参考文献:名称:EXTERNAL PREPARATIONS FOR SKIN摘要:公开号:EP1550429B1
文献信息
-
Nucleophilic Substitutions of Alcohols in High Levels of Catalytic Efficiency作者:Tanja Stach、Julia Dräger、Peter H. HuyDOI:10.1021/acs.orglett.8b01023日期:2018.5.18A practical method for the nucleophilic substitution (SN) of alcohols furnishing alkyl chlorides, bromides, and iodides under stereochemical inversion in high catalytic efficacy is introduced. The fusion of diethylcyclopropenone as a simple Lewis base organocatalyst and benzoyl chloride as a reagent allows notable turnover numbers up to 100. Moreover, the use of plain acetyl chloride as a stoichiometric
-
Esterification of Tertiary Amides by Alcohols Through C−N Bond Cleavage over CeO <sub>2</sub>作者:Takashi Toyao、Md. Nurnobi Rashed、Yoshitsugu Morita、Takashi Kamachi、S. M. A. Hakim Siddiki、Md. A. Ali、A. S. Touchy、Kenichi Kon、Zen Maeno、Kazunari Yoshizawa、Ken‐ichi ShimizuDOI:10.1002/cctc.201801098日期:2019.1.9process proceeds through rate limiting addition of a CeO2 lattice oxygen to the carbonyl group of the adsorbed acetamide species with energy barrier of 17.0 kcal/mol. This value matches well with experimental value (17.9 kcal/mol) obtained from analysis of the Arrhenius plot. Further studies by in situ FT‐IR and temperature programmed desorption using probe molecules demonstrate that both acidic and已经发现CeO 2促进叔酰胺形成酯的醇解反应。本催化体系操作简单,可回收,并且不需要添加剂。酯化过程显示出较宽的底物范围(> 45个实例;分离产率高达93%)。密度泛函理论(DFT)研究与现场FT-IR观察相结合的结果表明,该过程通过速率限制将CeO 2晶格氧加到吸附的乙酰胺类化合物的羰基上进行,能量垒为17.0 kcal / mol 。该值与通过分析Arrhenius图获得的实验值(17.9 kcal / mol)很好地匹配。原位进一步研究FT-IR和使用探针分子的程序升温脱附表明,酸性和碱性性质均很重要,因此,CeO 2对CN键断裂反应表现出最佳性能。
-
Metal-Free Carbonylations by Photoredox Catalysis作者:Michal Majek、Axel Jacobi von WangelinDOI:10.1002/anie.201408516日期:2015.2.9reaction driven by visible light and catalyzed by eosin Y which affords alkyl benzoates from arene diazonium salts, carbon monoxide, and alcohols under mild conditions. Tertiary esters can also be prepared in high yields. DFT calculations and radical trapping experiments support a catalytic photoredox pathway without the requirement for sacrificial redox partners.
-
Formamides as Lewis Base Catalysts in S<sub>N</sub>Reactions-Efficient Transformation of Alcohols into Chlorides, Amines, and Ethers作者:Peter H. Huy、Sebastian Motsch、Sarah M. KapplerDOI:10.1002/anie.201604921日期:2016.8.16and waste‐balance (E‐factor down to 2). Chiral substrates are converted with excellent levels of stereochemical inversion (99 %→≥95 % ee). In a practical one‐pot procedure, the primary formed chlorides can be further transformed into amines, azides, ethers, sulfides, and nitriles. The value of the method was demonstrated in straightforward syntheses of the drugs rac‐Clopidogrel and S‐Fendiline.
-
A General Catalytic Method for Highly Cost‐ and Atom‐Efficient Nucleophilic Substitutions作者:Peter H. Huy、Isabel FilbrichDOI:10.1002/chem.201800588日期:2018.5.23A general formamide‐catalyzed protocol for the efficient transformation of alcohols into alkyl chlorides, which is promoted by substoichiometric amounts (down to 34 mol %) of inexpensive trichlorotriazine (TCT), is introduced. This is the first example of a TCT‐mediated dihydroxychlorination of an OH‐containing substrate (e.g., alcohols and carboxylic acids) in which all three chlorine atoms of TCT
表征谱图
-
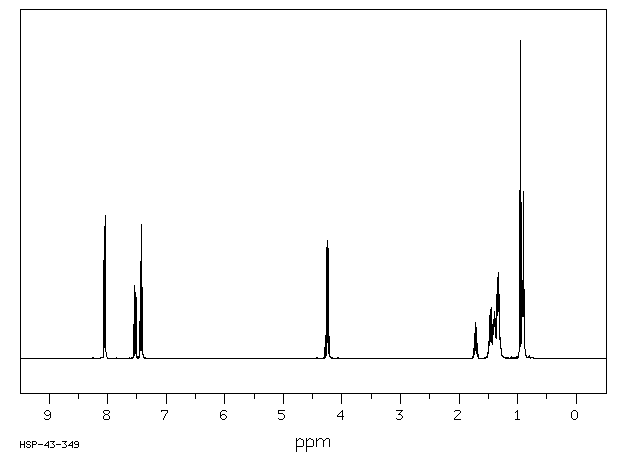
氢谱1HNMR
-
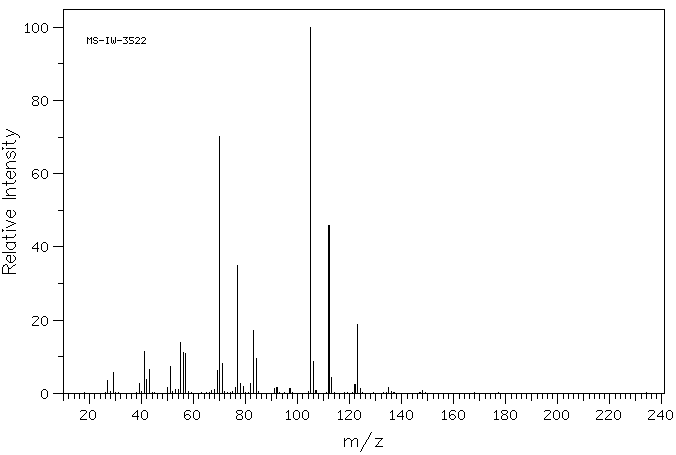
质谱MS
-
碳谱13CNMR
-
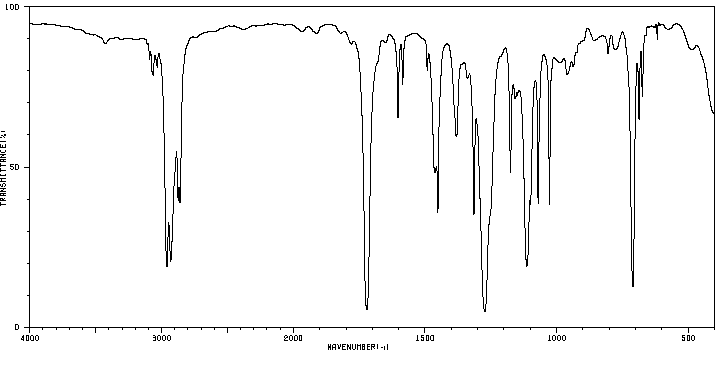
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫