3,3a,4,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,7-dione | 54022-56-9
中文名称
——
中文别名
——
英文名称
3,3a,4,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,7-dione
英文别名
rac-9,10-methanediyldioxy-galanth-15-ene-1,7-dione;3,3a,4,5-Tetrahydro-9,10-methylendioxy-pyrrolo<3,2,1-de>phenanthridin-1,7(2H)-dion;Caranine-7-one, 15,16-didehydro-1-oxo-;5,7-dioxa-12-azapentacyclo[10.6.1.02,10.04,8.015,19]nonadeca-1(19),2,4(8),9-tetraene-11,18-dione
CAS
54022-56-9
化学式
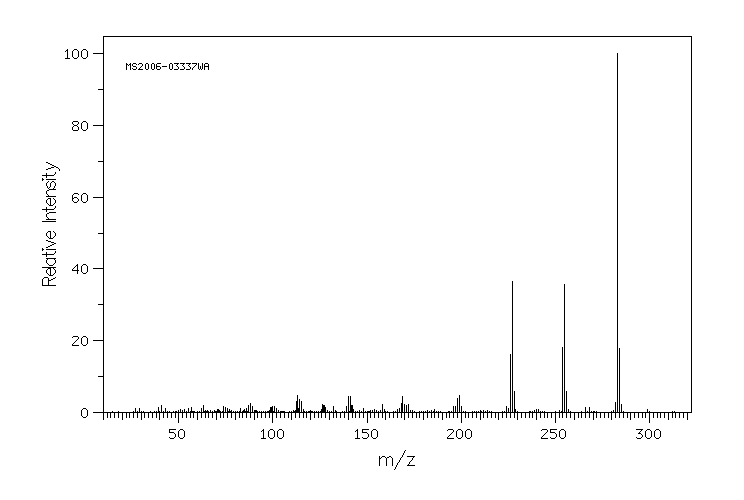
C16H13NO4
mdl
——
分子量
283.284
InChiKey
XOHQQVGGYQHPKV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:249-250 °C
-
沸点:543.2±50.0 °C(Predicted)
-
密度:1.53±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:21
-
可旋转键数:0
-
环数:5.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:55.8
-
氢给体数:0
-
氢受体数:4
上下游信息
反应信息
-
作为反应物:描述:3,3a,4,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,7-dione 在 platinum(IV) oxide 、 lithium aluminium tetrahydride 、 氢气 、 溶剂黄146 作用下, 以 四氢呋喃 为溶剂, 反应 114.0h, 生成 (+/-)-γ-lycorane参考文献:名称:通过顺序 C-H 功能化策略实现石蒜碱生物碱的发散全合成摘要:利用 Pd 催化的一锅连续 C-H 官能化策略从常见中间体4制备了四种石蒜碱生物碱和一种假石蒜碱生物碱。基于Pd(PPh 3 ) 4 /K 2 CO 3 /甲苯催化体系,通过切换空气、DMSO/H 2 O/I 2和DMSO/O 2氧化条件,得到三种关键中间体12a 、 12b ,可以通过良好控制的方式获得具有不同取代模式的12c和12c 。结果,通过该不同路线,在10个步骤内成功合成了四种天然产物γ-lycorane、hippadine、anHydrolycorinone和anHydrolycorine以及拟-lycorine生物碱Δ (4a,10b) -6-oxodihydrolycorine。DOI:10.1021/acs.joc.4c00054
-
作为产物:描述:5-溴-6-氯甲基-1,3-苯并间二氧杂环戊烯 在 氢氧化钾 、 氧气 、 苯基锂 、 二乙胺 作用下, 以 乙醚 、 乙醇 、 甲苯 为溶剂, 生成 3,3a,4,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,7-dione参考文献:名称:Ring closure of enaminones via intramolecular electrophilic arylation involving benzyne intermediates and its use in the synthesis of .gamma.-lycoran and related compounds摘要:DOI:10.1021/jo01321a010
文献信息
-
Intramolecular photochemical arylation of N-substituted enaminones: application to synthesis of heterocyclic compounds作者:Hideo Iida、Yoshifumi Yuasa、Chihiro KibayashiDOI:10.1039/c39780000766日期:——3-aminocyclohex-2-enones and 1,2,3,3a,4,5-hexahydro-1-(2-halogeno-4,5-methylenedioxybenzyl)indol-6-ones (10) and (11) underwent photochemical cyclisation to yield the five- to seven-membered heterocyclic compounds (5) and the 3,3a,4,5-tetrahydropyrrolo[3,2,1-de]phenanthridine-1,7-(2H)-dione (13), respectively.
-
Synthesis of 1,12b-didehydrolycoran (α-anhydrodihydrocaranine) and 12bα-lycoran (γ-lycoran)via photocyclisation of an enamide-ketone作者:Hideo Iida、Sakae Aoyagi、Chihiro KibayashiDOI:10.1039/p19750002502日期:——A novel stereoselective synthesis of lycorine-type alkaloids from a key intermediate obtained by photocyclisation of an enamido-ketone is described. Birch reduction of 6-methoxyindoline, prepared by a benzyne reaction of 3-chloro-4-methoxyphenethylamine, gave 3,3a,4,5-tetrahydro-6-methoxy-2H-indole (18) which reacted with benzoyl and 3,4-methylenedioxybenzoyl chloride to form the corresponding N-acylindol-6-one
-
A Practical Approach to 6H-Indol-6-ones Enables the Formal Synthesis of γ-Lycorane作者:Rui Zhan、Li-Dong Shao、Yang Chen、Xin-Ting Hu、Xiao-Yan Xie、Dashan Li、Chun-Xia Zheng、You-Xi Zhang、Wen-Jing WangDOI:10.1055/a-1878-8597日期:2023.1We represented herein a two-step synthesis of 1-methyl-6H-indol-6-one which is an N-containing 6/5 fused bicyclic building blocks in Amaryllidaceae alkaloids. The key step featured is a ‘one-pot’ ozonolysis/reductive amination/cyclization of allylated cyclohexa-1,3-dione to give bicyclic compounds. Moreover, the formal total synthesis of natural product γ-lycorane could be achieved through a photo-promoted
-
Exploitation of intramolecular photochemical arylation of N-substituted enaminones. Efficient, general synthesis of heterocyclic compounds作者:Hideo Iida、Yoshifumi Yuasa、Chihiro KibayashiDOI:10.1021/jo01322a009日期:1979.4
-
Iida,H. et al., Heterocycles, 1977, vol. 6, p. 1747 - 1751作者:Iida,H. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
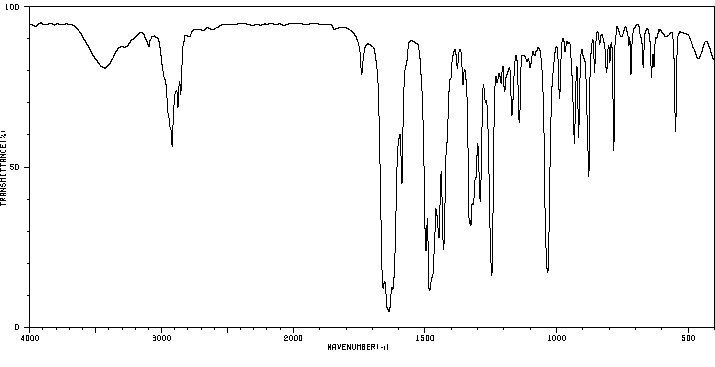
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43