双(3-氯苄)胺 | 129041-31-2
中文名称
双(3-氯苄)胺
中文别名
N,N-二(3-氯苄基)胺
英文名称
bis(3-chlorobenzyl)amine
英文别名
N,N-Bis(3-chlorobenzyl)amine;1-(3-chlorophenyl)-N-[(3-chlorophenyl)methyl]methanamine
CAS
129041-31-2
化学式
C14H13Cl2N
mdl
MFCD00671501
分子量
266.17
InChiKey
DNKUGPCGNWRIJJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S26
-
危险类别码:R36/37/38
-
海关编码:2921499090
SDS
上下游信息
反应信息
-
作为反应物:描述:双(3-氯苄)胺 在 sodium azide 、 2,4,6-trimethyl-N-[4-(2,4,6-trimethylphenyl)iminopent-2-en-2-yl]aniline 、 三乙胺 、 iron(II) chloride 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 12.08h, 生成 3-(3-chlorobenzyl)-2-(3-chlorophenyl)-5,5-dimethylimidazolidin-4-one参考文献:名称:铁催化的α-叠氮基酰胺的分子内CH胺化反应。摘要:最近,铁催化的脂肪族叠氮化物分子内CH胺化反应已成为制备氮杂环的有力工具。这篇论文报道了α-叠氮基酰胺可以通过分子内的C(sp3)-H胺化反应,由FeCl2和β-二酮配体组成的简单催化体系高效地转化为咪唑啉酮化合物。该反应为多取代的咪唑啉酮提供了一种简单且原子经济的方法。DOI:10.1021/acs.orglett.8b03927
-
作为产物:描述:3-氯苯腈 在 potassium tert-butylate 、 1,3,2-oxazaborolidine-borane 、 copper(II) bis(trifluoromethanesulfonate) 作用下, 以 水 为溶剂, 反应 24.0h, 以87%的产率得到双(3-氯苄)胺参考文献:名称:温和条件下铜催化腈转移加氢生成伯胺-硼烷和仲胺的选择性切换摘要:报道了一种简单而有效的铜催化腈选择性转移氢化为伯胺-硼烷和仲胺与恶氮硼烷-BH 3 配合物的方法。通过切换溶剂和铜催化剂在温和条件下实现选择性控制。通过该策略以高选择性和高达 95% 的产率合成了 30 多种伯胺-硼烷和 40 种仲胺。该策略应用于以89% 的收率合成15 N 标记。DOI:10.1021/acs.joc.1c02413
文献信息
-
Phosphine-Free Manganese Catalyst Enables Selective Transfer Hydrogenation of Nitriles to Primary and Secondary Amines Using Ammonia–Borane作者:Koushik Sarkar、Kuhali Das、Abhishek Kundu、Debashis Adhikari、Biplab MajiDOI:10.1021/acscatal.0c05406日期:2021.3.5primary and secondary amines by nitrile hydrogenation, employing a borrowing hydrogenation strategy. A class of phosphine-free manganese(I) complexes bearing sulfur side arms catalyzed the reaction under mild reaction conditions, where ammonia–borane is used as the source of hydrogen. The synthetic protocol is chemodivergent, as the final product is either primary or secondary amine, which can be controlled
-
2D sp <sup>2</sup> Carbon‐Conjugated Porphyrin Covalent Organic Framework for Cooperative Photocatalysis with TEMPO作者:Ji‐Long Shi、Rufan Chen、Huimin Hao、Cheng Wang、Xianjun LangDOI:10.1002/anie.202000723日期:2020.6.22D covalent organic frameworks (COFs) are receiving ongoing attention in semiconductor photocatalysis. Herein, we present a photocatalytic selective chemical transformation by combining sp2 carbon-conjugated porphyrin-based covalent organic framework (Por-sp2 c-COF) photocatalysis with TEMPO catalysis illuminated by 623 nm red light-emitting diodes (LEDs). Highly selective conversion of amines into
-
Radical 1,4‐Aryl Migration Enabled Remote Cross‐Electrophile Coupling of α‐Amino‐β‐Bromo Acid Esters with Aryl Bromides作者:Shi Tang、Zhen‐Hua Xu、Ting Liu、Shuo‐Wen Wang、Jian Yu、Jian Liu、Yu Hong、Shi‐Lu Chen、Jin He、Jin‐Heng LiDOI:10.1002/anie.202106273日期:2021.9.20two new C(sp3)−C(sp2) bonds using a bench-stable Ni/bipyridine/Zn system featuring a broad substrate scope and excellent diastereoselectivity, which provides an effective platform for the remote aryl group migration and arylation of amino acid esters via redox-neutral C(sp3)−C(sp2) bond cleavage. Mechanistically, this cascade reaction is accomplished by combining two powerful catalytic cycles consisting我们报告了一种前所未有的、高效的镍催化自由基中继,用于通过 1,4-芳基迁移/芳基化级联将 β-溴-α-苄基氨基酸酯与芳基溴进行远程交叉亲电偶联。β-溴-α-苄基氨基酸酯被认为是独特的分子支架,允许芳基迁移反应,这是自由基休战-微笑重排的概念新变体。该反应能够使用具有广泛底物范围和优异非对映选择性的长期稳定的 Ni/联吡啶/Zn 系统形成两个新的 C(SP 3 )-C(SP 2 ) 键,为远程芳基提供了一个有效的平台氨基酸酯通过氧化还原中性 C(SP 3 )-C(SP 2 ) 的迁移和芳基化) 键断裂。机械地,该级联反应由通过C(SP的产生相结合自由交亲电耦合和自由基1,4-芳迁移的两个强大的催化循环实现3)-centred从C的均裂自由基中间体(SP 3) -Br 键和瞬态烷基自由基转换为强大的 α-氨基烷基自由基。
-
Selective Ruthenium-Catalyzed Alkylation of Indoles by Using Amines作者:Sebastian Imm、Sebastian Bähn、Annegret Tillack、Kathleen Mevius、Lorenz Neubert、Matthias BellerDOI:10.1002/chem.200903261日期:——Lend me your hydrogen! The first transition‐metal‐catalyzed alkylations of indoles with aliphatic amines proceeding under transfer hydrogenation conditions are developed (see scheme). This reaction is based on the borrowing hydrogen methodology.
-
Catalytic Enantioselective Intramolecular C(sp <sup>3</sup> )−H Amination of 2‐Azidoacetamides作者:Zijun Zhou、Shuming Chen、Jie Qin、Xin Nie、Xingwen Zheng、Klaus Harms、Radostan Riedel、K. N. Houk、Eric MeggersDOI:10.1002/anie.201811927日期:2019.1.21An enantioselective ring‐closing C(sp3)−H amination of 2‐azidoacetamides is catalyzed by a chiral‐at‐metal ruthenium complex and provides chiral imidazolidin‐4‐ones in 31–95 % yield, with enantioselectivities of up to 95 % ee, and at catalyst loadings down to 0.1 mol % (turnover number (TON)=740). To our knowledge, this is the first example of a highly enantioselective C(sp3)−H amination with aliphatic
表征谱图
-
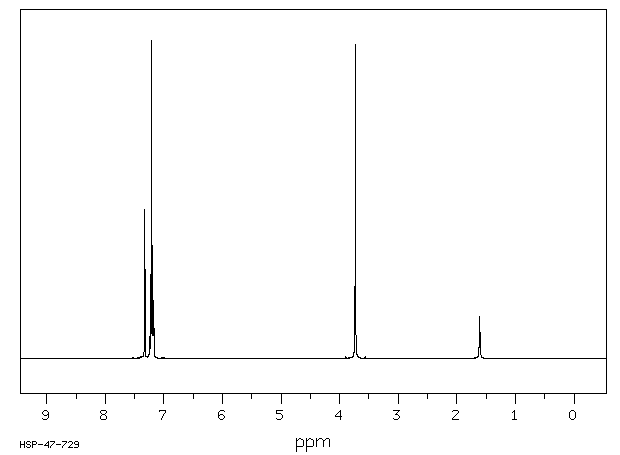
氢谱1HNMR
-
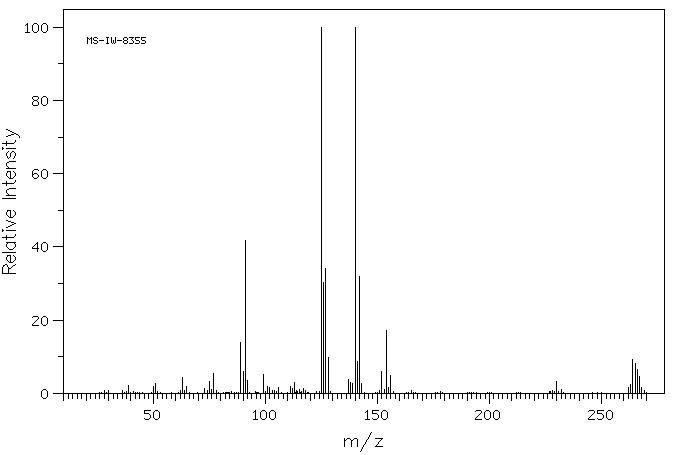
质谱MS
-
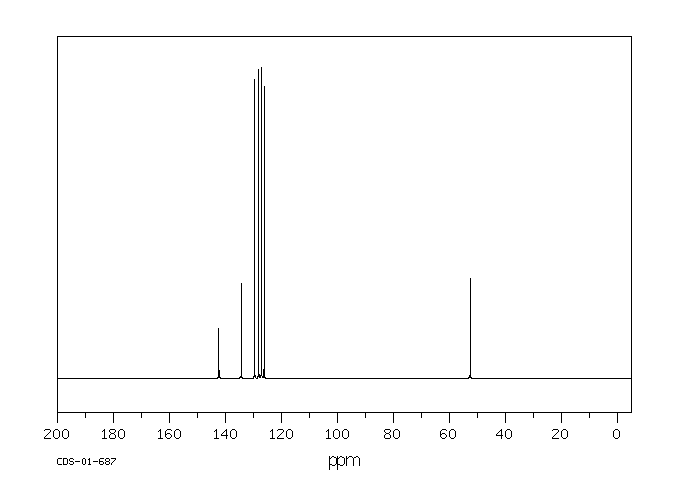
碳谱13CNMR
-
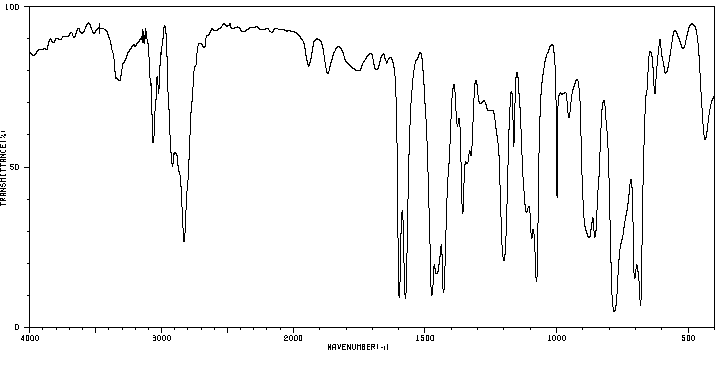
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫