三环[6.2.1.0(2,7)]undeca-4-ene | 91465-71-3
中文名称
三环[6.2.1.0(2,7)]undeca-4-ene
中文别名
三环[6.2.1.02,7]十一碳-4-烯;三环[6.2.1.0(2,7)]十一碳-4-烯
英文名称
tricyclo[6.2.1.02,7]undeca-4-ene
英文别名
tricyclo[6.2.1.02.7]undec-4-ene;tricyclo[6.2.1.02,7]undec-4-ene
CAS
91465-71-3
化学式
C11H16
mdl
MFCD00153968
分子量
148.248
InChiKey
YPOHZFOZYNRWKX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:209 °C
-
密度:0.96
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.818
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
储存条件:室温
SDS
三环[6.2.1.02,7]十一碳-4-烯
模块 1. 化学品
Tricyclo[6.2.1.02,7]undeca-4-ene
产品名称:
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三环[6.2.1.02,7]十一碳-4-烯
百分比: ....
CAS编码: 91465-71-3
分子式: C11H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
三环[6.2.1.02,7]十一碳-4-烯
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 209 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.96
三环[6.2.1.02,7]十一碳-4-烯
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
三环[6.2.1.02,7]十一碳-4-烯
模块16 - 其他信息
N/A
模块 1. 化学品
Tricyclo[6.2.1.02,7]undeca-4-ene
产品名称:
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三环[6.2.1.02,7]十一碳-4-烯
百分比: ....
CAS编码: 91465-71-3
分子式: C11H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
三环[6.2.1.02,7]十一碳-4-烯
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 209 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.96
三环[6.2.1.02,7]十一碳-4-烯
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
三环[6.2.1.02,7]十一碳-4-烯
模块16 - 其他信息
N/A
制备方法与用途
抗生素是一种用于治疗细菌感染的药物。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三环<6.2.1.02,7>十一烷 tricyclo<6.2.1.02,7>undecane 28691-42-1 C11H18 150.264
反应信息
-
作为反应物:描述:三环[6.2.1.0(2,7)]undeca-4-ene 在 palladium 10% on activated carbon 氢气 作用下, 以 甲醇 为溶剂, 20.0~70.0 ℃ 、4.24 MPa 条件下, 反应 1.25h, 以86%的产率得到三环<6.2.1.02,7>十一烷参考文献:名称:Polycyclic fluoroalkanes摘要:提供了对紫外波长范围从约190纳米到260纳米高度透明的多环氟烷。这些多环氟烷适用于各种应用,特别是在电磁波谱的真空紫外和深紫外区域。例如,这些多环氟烷在光学耦合剂、光学水泥、光学元件、半导体晶圆和器件的光学检测介质以及浸没光刻等方面非常有用,特别是在193和248纳米的曝光波长下。公开号:US07084314B1
-
作为产物:参考文献:名称:Polycyclic fluoroalkanes摘要:提供了对紫外波长范围从约190纳米到260纳米高度透明的多环氟烷。这些多环氟烷适用于各种应用,特别是在电磁波谱的真空紫外和深紫外区域。例如,这些多环氟烷在光学耦合剂、光学水泥、光学元件、半导体晶圆和器件的光学检测介质以及浸没光刻等方面非常有用,特别是在193和248纳米的曝光波长下。公开号:US07084314B1
文献信息
-
Palladium-Catalyzed Aerobic Oxidative Dehydrogenation of Cyclohexenes to Substituted Arene Derivatives作者:Andrei V. Iosub、Shannon S. StahlDOI:10.1021/ja512770u日期:2015.3.18system has been identified for aerobic dehydrogenation of substituted cyclohexenes to the corresponding arene derivatives. Use of sodium anthraquinone-2-sulfonate (AMS) as a cocatalyst enhances the product yields. A wide range of functional groups are tolerated in the reactions, and the scope and limitations of the method are described. The catalytic dehydrogenation of cyclohexenes is showcased in an efficient
-
Regio‐ and Stereoselective Thianthrenation of Olefins To Access Versatile Alkenyl Electrophiles作者:Junting Chen、Jiakun Li、Matthew B. Plutschack、Florian Berger、Tobias RitterDOI:10.1002/anie.201914215日期:2020.3.27we report a regioselective alkenyl electrophile synthesis from unactivated olefins that is based on a direct and regioselective C-H thianthrenation reaction. The selectivity is proposed to arise from an unusual inverse-electron-demand hetero-Diels-Alder reaction. The alkenyl sulfonium salts can serve as electrophiles in palladium- and ruthenium-catalyzed cross-coupling reactions to make alkenyl C-C,
-
Electrochemical vicinal aminotrifluoromethylation of alkenes: high regioselective acquisition of β-trifluoromethylamines作者:Yubing Huang、Huanliang Hong、Zirong Zou、Chunshu Liao、Jingjun Lu、Yongwei Qin、Yibiao Li、Lu ChenDOI:10.1039/c9ob00717b日期:——A novel vicinal aminotrifluoromethylation of alkenes using CF3SO2Na as a trifluoromethyl precursor and acetonitrile as an N-nucleophile has been achieved by an electrooxidative strategy. The present electrochemical protocol achieves efficient and highly regioselective difunctionalization of CC bonds under metal-free and external oxidant-free electrolysis conditions, leading to a series of β-trifluoromethylamine
-
Selective Difunctionalization of Unactivated Aliphatic Alkenes Enabled by a Metal–Metallaaromatic Catalytic System作者:Fei-Hu Cui、Yuhui Hua、Yu-Mei Lin、Jiawei Fei、Le-Han Gao、Xiaodan Zhao、Haiping XiaDOI:10.1021/jacs.1c12586日期:2022.2.9provided. These reactions produce 1,2-difunctionalized products with good yields and high levels of chemo-, regio-, and stereoselectivity. Our studies revealed the following: (i) The usually inert osmium center activates the N- or O-centered nucleophiles. (ii) The copper–osmium bonding and its cooperative effects play essential roles in control the selectivity by bringing the reaction components into有机金属催化剂的设计对于催化反应的发展至关重要。在此,我们描述了一种具有双金属、金属芳烃和钳形配合物特征的异金属[Os-Cu]配合物。该配合物可作为一种高效催化剂,用于未活化烯烃的选择性氨基和氧硒化。提供了 80 多个示例,包括此类反应中具有挑战性的不对称脂肪族烯烃和胺基亲核试剂的底物。这些反应产生具有良好收率和高水平化学、区域和立体选择性的 1,2-双官能化产物。我们的研究揭示了以下内容:(i)通常惰性的锇中心激活N - 或O- 中心的亲核试剂。(ii) 铜-锇键及其协同效应通过使反应组分靠近,在控制选择性方面发挥着重要作用。(iii) 金属芳族部分有助于稳定中间体。这些发现为基于金属-金属芳烃协同效应的催化剂设计提供了一个多功能平台,这是以前用双金属配合物无法实现的。
-
[EN] ESTER COMPOUND<br/>[FR] COMPOSÉ ESTER<br/>[JA] エステル化合物申请人:MITSUI CHEMICALS INC公开号:WO2022045231A1公开(公告)日:2022-03-03本発明のエステル化合物は下記式(1)で表される〔式中、R1~R24は、それぞれ独立に水素原子、ハロゲン原子、炭化水素基またはヘテロ原子含有炭化水素基である。R1~R10、R23およびR24は互いに結合して環を形成してもよく、隣接する置換基が直接結合した多重結合を形成してもよい。R11~R24は互いに結合して環を形成してもよく、隣接する置換基が互いに結合して多重結合を形成してもよい。R1~R24において少なくとも1組は互いに結合して環構造を形成する。n2~n5は、それぞれ独立に0~2の整数を表す。n1およびn6は、それぞれ独立に0または1の整数を表す。L1およびL2は、それぞれ独立に炭化水素基またはヘテロ原子含有炭化水素基である。〕。这项发明的酯化合物由以下式(1)表示[式中,R1-R24分别独立地是氢原子、卤素原子、烃基或含有杂原子的烃基。 R1-R10、R23和R24可以相互连接形成环,相邻的取代基也可以直接连接形成多重键。 R11-R24可以相互连接形成环,相邻的取代基也可以相互连接形成多重键。 在R1-R24中,至少有一对相互连接形成环结构。 n2-n5分别独立地表示0-2的整数。 n1和n6分别独立地表示0或1的整数。 L1和L2分别独立地是烃基或含有杂原子的烃基。]。
表征谱图
-
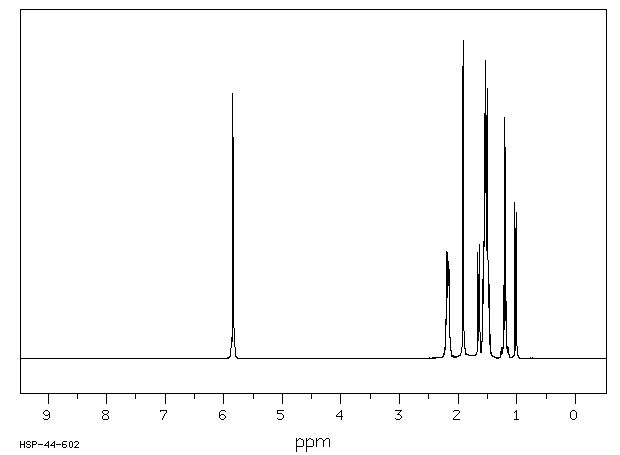
氢谱1HNMR
-
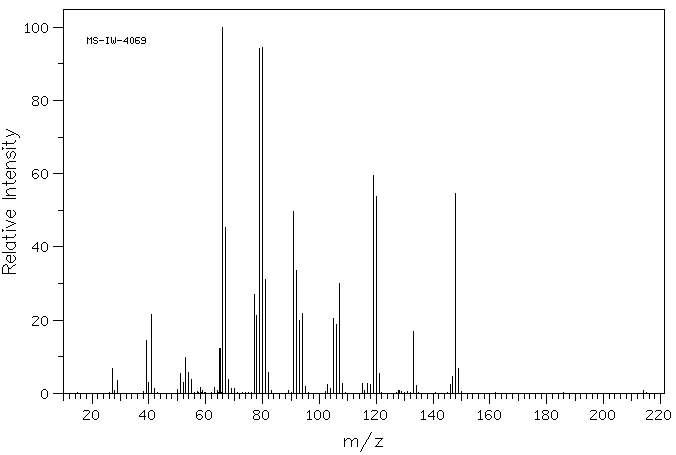
质谱MS
-
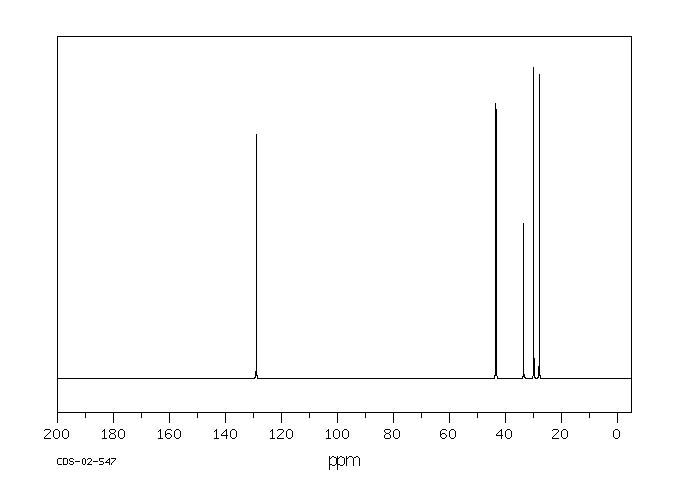
碳谱13CNMR
-
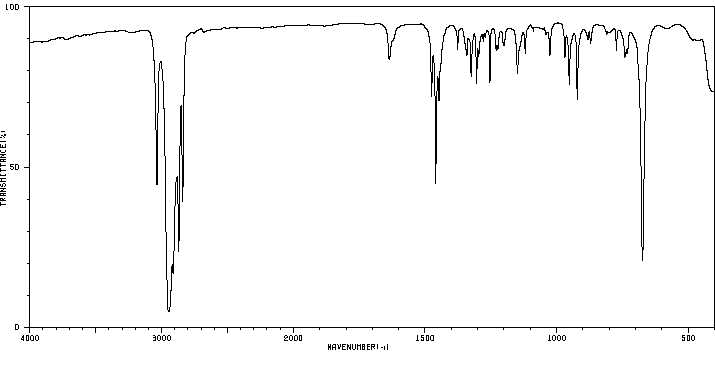
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸