降冰片烯 | 498-66-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:44-46 °C (lit.)
-
沸点:96 °C (lit.)
-
密度:0.955
-
闪点:5 °F
-
溶解度:可溶于氯仿、乙酸乙酯(微量)、甲醇(微量)
-
暴露限值:ACGIH: TWA 20 ppmOSHA: Ceiling 300 ppm; TWA 200 ppmNIOSH: IDLH 500 ppm; TWA 100 ppm(375 mg/m3); STEL 150 ppm(560 mg/m3)
-
LogP:4.101 at 25℃
-
蒸汽压力:39.19 mmHg
-
大气OH速率常数:4.93e-11 cm3/molecule*sec
-
保留指数:726;731;726;731;699;727
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:7
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:4.1
-
危险品标志:F
-
安全说明:S16,S29,S33,S9
-
危险类别码:R11
-
WGK Germany:1
-
海关编码:29021990
-
危险品运输编号:UN 1325 4.1/PG 2
-
危险类别:4.1
-
RTECS号:RB7900000
-
包装等级:II
-
危险标志:GHS02,GHS07,GHS09
-
危险性描述:H228,H319,H411
-
危险性防范说明:P210,P273,P305 + P351 + P338
-
储存条件:本品应远离火源,并在通风良好的地方密封保存。
SDS
| Name: | Norbornylene stabilized 99% Material Safety Data Sheet |
| Synonym: | Bicyclo[2.2.1]-2-heptene; Norbornene; Norbornylene; Bicyclo[2.2.1]hept-2-ene |
| CAS: | 498-66-8 |
Synonym:Bicyclo[2.2.1]-2-heptene; Norbornene; Norbornylene; Bicyclo[2.2.1]hept-2-ene
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 498-66-8 | Norbornylene | 99 | 207-866-0 |
Risk Phrases: 11
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable.Extremely flammable.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors can travel to a source of ignition and flash back. Flammable solid.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Remove all sources of ignition. Provide ventilation. Use only non-sparking tools and equipment.
Section 7 - HANDLING and STORAGE
Handling:
Use spark-proof tools and explosion proof equipment. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Take precautionary measures against static discharges. Use only with adequate ventilation. Keep away from heat, sparks and flame.
Storage:
Keep away from sources of ignition. Store in a tightly closed container. Refrigerator/flammables.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 498-66-8: Russia: 3 mg/m3 TWA Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: pungent sour
pH: Not available.
Vapor Pressure: 39.2 mm Hg @ 25 deg C
Viscosity: Not available.
Boiling Point: 96 deg C @ 760 mm Hg
Freezing/Melting Point: 42 - 46 deg C
Autoignition Temperature: Not available.
Flash Point: -15 deg C ( 5.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: practically insoluble
Specific Gravity/Density:
Molecular Formula: C7H10
Molecular Weight: 94.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
No information found.
Conditions to Avoid:
High temperatures, ignition sources, confined spaces.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: May occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 498-66-8: RB7900000 LD50/LC50:
CAS# 498-66-8: Oral, mouse: LD50 = 13000 mg/kg; Oral, rat: LD50 = 11300 mg/kg; Skin, rabbit: LD50 = >5 mL/kg.
Carcinogenicity:
Norbornylene - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.*
Hazard Class: 4.1
UN Number: 1325
Packing Group: II
IMO
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.
Hazard Class: 4.1
UN Number: 1325
Packing Group: II
RID/ADR
Shipping Name: FLAMMABLE SOLID, ORGANIC, N.O.S.
Hazard Class: 4.1
UN Number: 1325
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F
Risk Phrases:
R 11 Highly flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 33 Take precautionary measures against static
discharges.
WGK (Water Danger/Protection)
CAS# 498-66-8: No information available.
Canada
CAS# 498-66-8 is listed on Canada's NDSL List.
CAS# 498-66-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 498-66-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
降冰片烯(NB)常温下为白色透明结晶,易升华。它主要用于合成环烯烃共聚物(COC)。COC具有低密度、低吸湿性、高透明性、高耐热性、高折光指数以及优良的加工性能等优势,在工业界受到高度关注。
应用降冰片烯是一种重要的共聚单体,可与乙烯、丙烯和苯乙烯等α-烯烃在Ziegler-Natta或茂金属催化剂的作用下通过非开环聚合反应制备环烯烃共聚物。这种材料兼具低密度、低吸湿性、高透明性、高耐热性和优良的加工性能,近年来在工业界和学术界引起了高度重视,并在光学、电子、医药包装等领域展现出广泛的应用前景。
制备一种降冰片烯的生产方法采用两个串联的第一釜式反应器和第二釜式反应器。具体步骤如下:
-
将配制好的双环戊二烯(DCPD)、溶剂甲基异丁基酮(MIBK)以及阻聚剂对叔丁基邻苯二酚(TBC)溶液,通过泵从第一釜式反应器的底部连续送入。开启搅拌后通入所需比例的乙烯气体,使乙烯溶解在溶剂中;其中乙烯与双环戊二烯(DCPD)的摩尔比为10~50:1,溶剂甲基异丁基酮(MIBK)与双环戊二烯(DCPD)的重量比为0.5~2.0:1,阻聚剂对叔丁基邻苯二酚(TBC)加入量为双环戊二烯(DCPD)质量的0.003%。反应温度控制在170~190℃之间,并通过乙烯压缩机持续补充反应消耗的乙烯以维持系统压力为7~11MPa,物料在第一釜式反应器中的停留时间为10~120分钟,在此期间连续排出至第二釜式反应器。
-
在第二釜式反应器中,继续控制反应温度为200~220℃,持续补充反应消耗的乙烯,使系统压力维持在7~10MPa之间,物料停留时间为10~80分钟。反应后的液体通过设置在反应器侧面的出料口连续排出至精制系统进行溶剂甲基异丁基酮(MIBK)回收和降冰片烯(NB)产品的精制。循环使用的溶剂可以多次利用。精制后得到的降冰片烯(NB)纯度可达99.9%以上。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲基二环[2.2.1]庚-2-烯 5-methyl-2-norbornene 822-96-8 C8H12 108.183 —— 5-hexyl-2-norbornene 22094-83-3 C13H22 178.318 —— 5-decylbicyclo[2.2.1]hept-2-ene 22094-85-5 C17H30 234.425 —— bicyclo<3.2.)>hept-2-ene 7095-65-0 C7H10 94.1564 —— tetracyclododecene 90242-59-4 C12H16 160.259 —— bicyclo[4.1.0]hept-2-ene 2566-57-6 C7H10 94.1564 2,5-降冰片二烯 bicyclo[2.2.1]hepta-2,5-diene 121-46-0 C7H8 92.1405 二聚环戊二烯 bi(cyclopentadiene) 77-73-6 C10H12 132.205 —— syn-7-bromobicyclo<2.2.1>heptene 60154-55-4 C7H9Br 173.052 —— endo-Dicyclopentadien 1755-01-7 C10H12 132.205 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3-Di-(α-propenyl)-cyclopentan 25172-65-0 C11H18 150.264 —— bicyclo[3.2.1]oct-2-ene 61617-43-4 C8H12 108.183 —— (1α,4α,4aβ,5α,8α,8aβ)-1,2,3,4,4a,5,8,8a-octahydro-1,4:5,8-dimethanonaphthalene 90243-18-8 C12H16 160.259 —— tetracyclododecene 90242-59-4 C12H16 160.259 —— exo,exo,endo,exo-hexacyclo<6.6.12,7.13,6.09,14.110,13>heptadec-4-ene 87424-91-7 C17H22 226.362 —— endo,anti-tetracyclododecene 15914-93-9 C12H16 160.259 —— cis-1,3-Divinylcyclopentan 10283-90-6 C9H14 122.21 —— 5-(5-bromopent-1-yl)bicyclo[2.2.1]hept-2-ene 511243-82-6 C12H19Br 243.187 3a,4,4a,5,8,8a,9,9a-八氢-4,9:5,8-二甲桥-1H-苯并[f]茚 endo,exo,endo-pentacyclo<6.5.1.02,7.13,6.09,13>pentadeca-4,10-diene 7158-25-0 C15H18 198.308 —— (Z)-3-((1R,3S)-3-vinylcyclopentyl)prop-2-en-1-ol —— C10H16O 152.236 —— exo-tricyclo<3.2.1.02,4>oct-6-ene 3635-95-8 C8H10 106.167 2,5-降冰片二烯 bicyclo[2.2.1]hepta-2,5-diene 121-46-0 C7H8 92.1405 —— syn-7-bromobicyclo<2.2.1>heptene 60154-55-4 C7H9Br 173.052 双环[2.2.1]-2-庚烯-7-醇 anti-bicyclo<2.2.1>hept-2-en-7-ol 53783-87-2 C7H10O 110.156 - 1
- 2
反应信息
-
作为反应物:描述:降冰片烯 在 吡啶 、 sodium amalgam 、 氘氧化钠 、 mercury(II) diacetate 作用下, 反应 87.0h, 生成 <3-exo-2H1>bicyclo<2.2.1>heptan-2-exo-yl toluene-p-sulphonate参考文献:名称:Stereochemistry of base-promoted 1,2-elimination from exo-2-bicyclo[2.2.1]heptyl tosylate and chloride摘要:Elimination reactions of exo-2-bicyclo[2.2.1]heptyl tosylate and chloride and their exo-3-deuterated analogues are studied in base-solvent systems that induce clean bimolecular 1,2-eliminations. The relative propensities for competitive syn-exo and anti-endo-H elimination modes are assessed from nonkinetically determined deuterium isotope effects and the deuterium content in the bicyclo[2.2.1]hept-2-ene formed from the deuterated substrates. The competition between syn-exo and anti-endo-H elimination is influenced by base association, which stabilizes the syn elimination transition state. Potential steric hindrance by oversized dissociated bases has no effect on the elimination stereochemistry.DOI:10.1021/jo00001a041
-
作为产物:参考文献:名称:催化前体中不对称还原前手性烯烃的二烯烃配体加氢的动力学研究[1]摘要:通过[Rh(L)(PP *)] A类型的手性铑(I)配合物(L = COD,[(Z,Z)-cycloocta-1,5-diene]或NBD(norborna-2; 5 -二烯),PP * =手性bisphosphane形成7元螯合环,A =阴离子等BF 4 - )往往与所造成的催化剂的部分遮挡的诱导期相关联。NBD配合物的氢化速度快于相应的COD配合物。COD / NBD混合物的催化加氢和米氏常数的确定表明,COD配合物在氢下的稳态浓度高于NBD配合物。但是,在氩气下,NBD配合物占主导地位,因为与COD配合物相比,NBD配合物具有更高的热力学稳定性。31 P-NMR谱。通过UV / Vis光谱法证明了在氢化条件下,热力学确定的COD与NBD配合物浓度之比的完全逆转。DOI:10.1002/cber.19961290117
-
作为试剂:描述:4-碘苯甲酸甲酯 、 3,5-双三氟甲基苄基溴 在 降冰片烯 、 三(2-呋喃基)膦 、 palladium diacetate 、 caesium carbonate 作用下, 以 四氢呋喃 、 异丙醇 、 甲苯 为溶剂, 以47 %的产率得到参考文献:名称:由钯/降冰片烯催化实现的内部功能化树枝状大分子的模块化合成平台摘要:内部功能化树枝状大分子的合成通常是繁琐且劳动密集型的,这一直是阻碍其实际应用的关键因素。在这里,我们重新审视了这一长期存在的挑战,并设计了一个模块化聚合平台,通过原位功能化策略合成具有全碳主链的多功能树枝,最多可达四代。通过钯/降冰片烯协同催化,在树枝状大分子生长过程中可以将不同的官能团引入到每一代树枝状大分子中,从而方便系统地比较它们的性能。这种多功能平台的实用性进一步体现在树枝状分子作为有机凝胶和聚集诱导发射材料的内部功能化依赖性特性中。DOI:10.1021/jacs.4c06090
文献信息
-
Catalytic Azido‐Hydrazination of Alkenes Enabled by Visible Light: Mechanistic Studies and Synthetic Applications作者:Peng Wang、Yunxuan Luo、Songsong Zhu、Dengfu Lu、Yuefa GongDOI:10.1002/adsc.201901041日期:2019.12.17visible‐light‐enabled catalytic intermolecular azido‐hydrazination method for unactivated alkenes is developed via an orderly radical addition sequence. This transformation features metal‐free and redox‐neutral conditions and is applicable to a wide range of alkenes with commercially available reagents. Mechanistic and kinetic studies reveal that the efficient generation of azide radical enabled by fluorenone
-
Development of the Copper-Catalyzed Olefin Aziridination Reaction作者:David A. Evans、Mark T. Bilodeau、Margaret M. FaulDOI:10.1021/ja00086a007日期:1994.4Soluble Cu(1) and Cu(I1) triflate and perchlorate salts are efficient catalysts for the aziridination of olefins employing (N-@-tolylsulfonyl)imino)phenyliodinane, PhI=NTs, as the nitrene precursor. Electron-rich as well as electron-deficient olefins undergo aziridination with this reagent in 55-958 yields, at temperatures ranging from -20 OC to +25 OC. The catalyzed nitrogen atom-transfer reaction可溶性 Cu(1) 和 Cu(I1) 三氟甲磺酸盐和高氯酸盐是使用(N-@-甲苯磺酰基)亚氨基)苯基碘烷(PhI=NTs)作为氮烯前体的烯烃氮丙啶化反应的有效催化剂。在 -20 OC 至 +25 OC 的温度范围内,富电子和缺电子烯烃与该试剂发生氮丙啶化反应,产率为 55-958。还开发了催化氮原子转移反应生成烯醇硅烷和甲硅烷基乙烯酮缩醛,以提供 α-氨基酮的简便合成。发现其他金属配合物在催化反应方面效果较差,而 PhI=NTs 被证明优于其他亚氨基供体作为氮烯前体。在极性非质子溶剂如 MeCN 和 MeNO 2 中反应速率和产率提高。对顺式和反式双取代烯烃的氮丙啶化反应的立体定向性进行了评估,发现它依赖于催化剂和底物。单和双取代烯烃对之间的分子间竞争实验表明,反应的烯烃选择性曲线与所用铜催化剂的氧化态无关。得出的结论是,在所用条件下,这些反应是通过 2+ 催化剂氧化态进行的。
-
Structure−Activity Relationships of Potent, Selective Inhibitors of Neuronal Nitric Oxide Synthase Based on the 6-Phenyl-2-aminopyridine Structure作者:John A. Lowe、Weimin Qian、Susan E. Drozda、Robert A. Volkmann、Deane Nason、Robert B. Nelson、Charles Nolan、Dane Liston、Karen Ward、Steve Faraci、Kim Verdries、Pat Seymour、Michael Majchrzak、Anabella Villalobos、W. Frost WhiteDOI:10.1021/jm030519g日期:2004.3.1The synthesis and structure-activity relationships of a series of 6-phenyl-2-aminopyridines that potently and selectively inhibit the neuronal isoform of nitric oxide synthase (nNOS) are described. Compound 14bi from this series exhibits potent in vivo activity in harmaline-induced cGMP formation in rat cerebellum, a functional model of nNOS inhibition, and in the PCP-induced hypermotility model in
-
Nitrogen-enriched porous carbon supported Pd-nanoparticles as an efficient catalyst for the transfer hydrogenation of alkenes作者:Jie Li、Xin Zhou、Ning-Zhao Shang、Cheng Feng、Shu-Tao Gao、Chun WangDOI:10.1039/c8nj03656j日期:——g-C3N4 as a nitrogen source and a self-sacrificial template. The prepared Pd@NPC exhibited superior catalytic activity and chemoselectivity for the catalytic transfer hydrogenation of alkenes under mild conditions with formic acid as a hydrogen donor. Moreover, the catalyst displays high structure stability, and can be reused for five runs without any significant decrease of its catalytic activity and
-
Tetrahydroxydiboron-Mediated Palladium-Catalyzed Transfer Hydrogenation and Deuteriation of Alkenes and Alkynes Using Water as the Stoichiometric H or D Atom Donor作者:Steven P. Cummings、Thanh-Ngoc Le、Gilberto E. Fernandez、Lorenzo G. Quiambao、Benjamin J. StokesDOI:10.1021/jacs.6b02132日期:2016.5.18There are few examples of catalytic transfer hydrogenations of simple alkenes and alkynes that use water as a stoichiometric H or D atom donor. We have found that diboron reagents efficiently mediate the transfer of H or D atoms from water directly onto unsaturated C-C bonds using a palladium catalyst. This reaction is conducted on a broad variety of alkenes and alkynes at ambient temperature, and boric
表征谱图
-
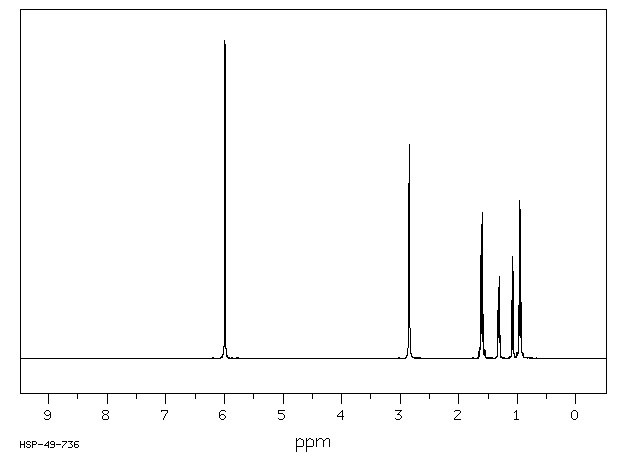
氢谱1HNMR
-
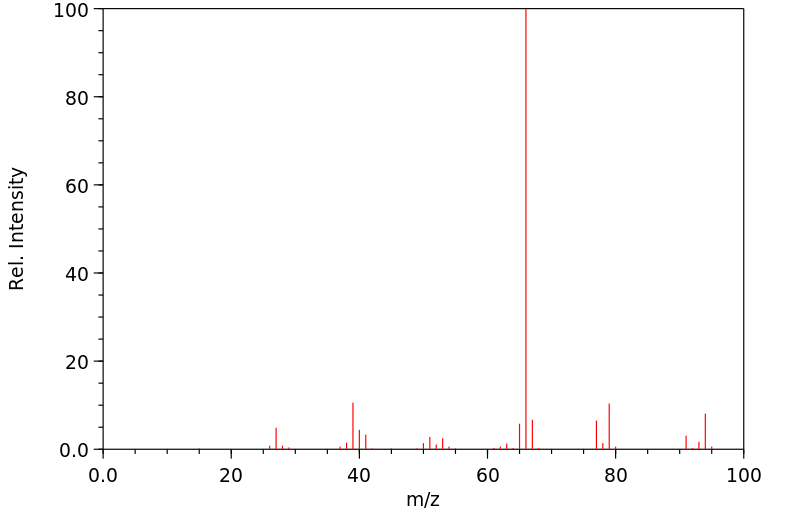
质谱MS
-
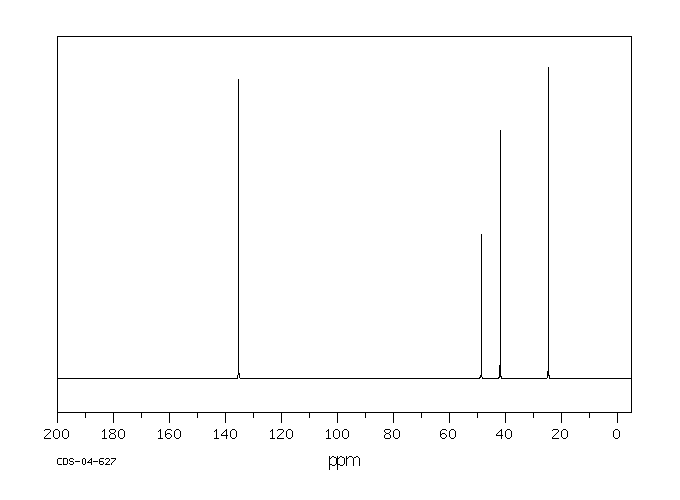
碳谱13CNMR
-
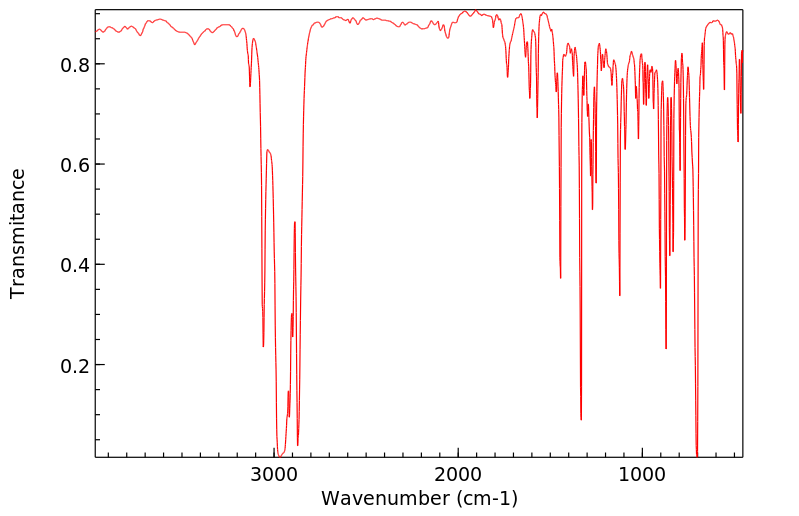
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息