N,N′-双(对甲苯磺酰)肼 | 14062-05-6
中文名称
N,N′-双(对甲苯磺酰)肼
中文别名
1,2-双(对甲苯磺酰)肼;2-[(4-甲基苯基)磺酰基]-4-甲苯磺酰肼;1,2-双(对甲苯基磺酰基)肼;N,N′-二甲苯磺酰肼;N,N'-二甲苯磺酰基肼
英文名称
N,N'-ditosylhydrazine
英文别名
4-methyl-N'-(p-tolylsulfonyl)benzenesulfonohydrazide;N,N′-ditosylhydrazine;4-methyl-N'-tosylbenzenesulfonohydrazide;N,N'-bis(p-toluenesulfonyl)hydrazine;N,N’-ditosyl hydrazine;4-methyl-N′-tosylbenzenesulfonohydrazide;N,N’-bis(p-toluenesulfonyl)hydrazine;1,2-bis(p-toluenesulfonyl)hydrazine;bis(p-toluenesulfonyl)hydrazine;1,2-ditosylhydrazine;4-methyl-N'-(4-methylphenyl)sulfonylbenzenesulfonohydrazide
CAS
14062-05-6
化学式
C14H16N2O4S2
mdl
——
分子量
340.424
InChiKey
CVRIWLRSJCDOOX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:220-224 °C
-
沸点:507.9±53.0 °C(Predicted)
-
密度:1.355±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:22
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:109
-
氢给体数:2
-
氢受体数:6
安全信息
-
危险品标志:Xi
-
安全说明:S26
-
危险类别码:R36/37/38
-
海关编码:2935009090
-
包装等级:III
-
危险类别:4.1
-
危险性防范说明:P280,P210,P240,P264,P270,P301+P310,P330,P370+P378,P403+P233,P405,P501
-
危险品运输编号:1325
-
危险性描述:H228,H302,H317,H319,H341,H351
-
储存条件:2-8°C,干燥密封保存。
SDS
上下游信息
反应信息
-
作为反应物:描述:N,N′-双(对甲苯磺酰)肼 在 硝酸 作用下, 反应 4.0h, 以52%的产率得到二-(4-甲苯基)-二砜参考文献:名称:Eine einfache und allgemeine Methode zur Herstellung von α-Disulfonen (R1SO2SO2R2)摘要:一种简便通用的α-二砜制备方法 α-二砜(R1SO2SO2R2)的二烷基、二芳基及烷基芳基二砜可以通过硝酸氧化N,N′-二磺酰肼来轻松制备。该方法适用于硫原子上多种取代基(R1,R2)。DOI:10.1055/s-1993-25891
-
作为产物:参考文献:名称:通过杂Diels-Alder反应合成α-氨基羰基化合物摘要:描述了通过杂 Diels-Alder 反应合成 α-氨基酮衍生物的途径。二酰基肼在吡啶存在下被次氯酸叔丁酯氧化。蒸发后,在不分离偶氮二羰基化合物的情况下进行与二烯的杂狄尔斯-阿尔德反应。当使用 Hf(OTf)4 或 AgOTf 作为催化剂时,使用 1 当量的二烯可以进行定量的杂 Diels-Alder 反应。产物的 N-N 键在 THF 中叔丁醇存在下通过 SmI2 还原被裂解。此外,C=C 双键的臭氧分解以优异的产率提供了 α-氨基酮衍生物。DOI:10.1246/cl.170970
-
作为试剂:描述:2-溴-1-(2,3-二氢-1H-吲哚-1-基)乙酮 在 tris-(dibenzylideneacetone)dipalladium(0) 、 N,N′-双(对甲苯磺酰)肼 、 四丁基溴化铵 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 双(2-二苯基磷苯基)醚 作用下, 以 四氢呋喃 、 1,2-二氯乙烷 为溶剂, 反应 18.0h, 生成 (6,6-difluoro-5-oxaspiro[2.4]heptan-7-yl)(indolin-1-yl)methanone参考文献:名称:钯催化螺环丙烷化合成偕二氟化 Oxa/Azaspiro[2.4]庚烷摘要:已成功开发出钯催化偕二氟烯烃与π-烯丙基钯1,4-偶极子的螺环丙烷化反应,为构建新型偕二氟螺环化合物6,6-二氟-5-提供了一种强大而简单的合成策略。氧杂/氮杂螺[2.4]庚烷。偕二氟烯烃的范围可以扩展到苯乙烯、丙烯酸酯和丙烯酰胺,实现在螺环骨架上安装各种官能团和不同的杂原子,从而转化为具有潜在生物活性的有价值的化合物。机理研究揭示了π-烯丙基钯两性离子中间体的螺环丙烷化和β-F消除之间的竞争。DOI:10.1021/acs.orglett.4c01912
文献信息
-
Photocatalyzed Coupling–Cyclization of <i>ortho</i>-Alkynylaryl Vinylethers with Arylsulfonyl Azides作者:Fengjuan Chen、Xiang Huang、Can Yang、Huanfeng Jiang、Wei ZengDOI:10.1021/acs.joc.1c01437日期:2021.11.5A novel visible-light-induced coupling–cyclization of ortho-alkynylaryl vinylethers with arylsulfonyl azides has been described. This transformation provided a concise approach to access C3-exocyclic C═C bond/C2-alkylsulfone-tethered benzofurans via a solvent-leveraged carbosulfonylation and [2 + 2 + 3] cyclization. Primary mechanistic studies demonstrated that THF belongs to a crucial H atom source
-
Asymmetric transfer hydrogenation of heterocycle-containing acetophenone derivatives using N-functionalised [(benzene)Ru(II)(TsDPEN)] complexes作者:Jonathan Barrios-Rivera、Yingjian Xu、Guy J. Clarkson、Martin WillsDOI:10.1016/j.tet.2021.132562日期:2022.1The application of enantiomerically-pure ruthenium(II) catalysts containing N - functionalised TsDPEN ligand to the asymmetric transfer hydrogenation of 15 examples of α-heterocyclic acetophenone derivatives is reported. Products of up to 99% ee were formed.
-
<i>N</i>,<i>N</i>‘-Ditosylhydrazine: A Convenient Reagent for Facile Synthesis of Diazoacetates作者:Tatsuya Toma、Jun Shimokawa、Tohru FukuyamaDOI:10.1021/ol701432k日期:2007.8.1A novel entry to the synthesis of diazoacetates is disclosed. A variety of diazoacetates were synthesized from the corresponding bromoacetates by treatment with N,N'-ditosylhydrazine in moderate to high yields. Ease of operation with the stable crystalline reagent as well as a short reaction time offer a useful alternative to the conventional methods.
-
Enantioselective Nucleophilic β-Carbon-Atom Amination of Enals: Carbene-Catalyzed Formal [3+2] Reactions作者:Xingxing Wu、Bin Liu、Yuexia Zhang、Martin Jeret、Honglin Wang、Pengcheng Zheng、Song Yang、Bao-An Song、Yonggui Robin ChiDOI:10.1002/anie.201606571日期:2016.9.26enantioselective β‐carbon amination for enals is disclosed. The nitrogen atom from a protected hydrazine with suitable electronic properties readily behaves as a nucleophile. Addition of the nitrogen nucleophile to a catalytically generated N‐heterocyclic‐carbene‐bound α,β‐unsaturated acyl azolium intermediate constructs a new carbon–nitrogen bond asymmetrically. The pyrazolidinone products from our catalytic
-
ALCOHOL DERIVATIVES AS KV7 POTASSIUM CHANNEL OPENERS申请人:H. Lundbeck A/S公开号:US20210032196A1公开(公告)日:2021-02-04The present invention provides novel compounds which activate the Kv7 potassium channels. Separate aspects of the invention are directed to pharmaceutical compositions comprising said compounds and uses of the compounds to treat disorders responsive to the activation of Kv7 potassium channels.
表征谱图
-
氢谱1HNMR
-
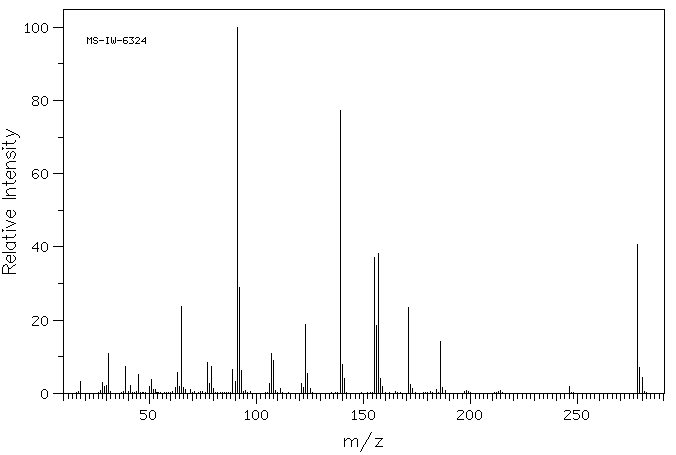
质谱MS
-
碳谱13CNMR
-
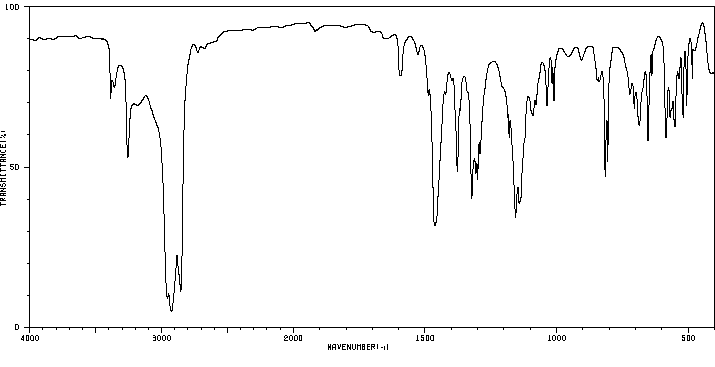
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫