sodium 3-aminobenzenesulfonate | 1126-34-7
中文名称
——
中文别名
——
英文名称
sodium 3-aminobenzenesulfonate
英文别名
sodium m-aminobenzenesulfonate;sodium;3-aminobenzenesulfonate
CAS
1126-34-7
化学式
C6H6NO3S*Na
mdl
——
分子量
195.174
InChiKey
GLXWXYTYBIBBLD-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):-2.82
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:91.6
-
氢给体数:1
-
氢受体数:4
安全信息
-
海关编码:2921420090
SDS
制备方法与用途
反应信息
-
作为反应物:描述:sodium 3-aminobenzenesulfonate 在 硝酸 、 magnesium oxide 、 sodium hydroxide 作用下, 以 水 为溶剂, 反应 6.5h, 生成 N,N-二丁基间氨基苯酚参考文献:名称:一种N,N-二丁基间氨基苯酚的制备方法摘要:本发明涉及一种N,N‑二丁基间氨基苯酚的制备方法,其包括以下步骤,1将间氨基苯磺酸钠、溶剂、卤代烃和缚酸剂进行烷基化反应,烷基化反应温度为80~200℃,烷基化反应时间为2~5小时,得到烷基化物的溶液;2将烷基化物的溶液和碱性试剂、水混合进行碱熔反应,碱熔反应温度为200~350℃,反应时间为1‑3小时得到烷基化的酚钠盐,然后再加入水;3将烷基化的酚钠盐与酸性试剂进行酸析反应,反应时间为0.5h‑3h,得到粗品N,N‑二丁基间氨基苯酚;4精制得到N,N‑二丁基间氨基苯酚;使用本发明方法制得的N,N‑二丁基间氨基苯酚收率高,成本低,减少了副产种类,减轻了后处理的负担。公开号:CN108558710B
-
作为产物:描述:参考文献:名称:[EN] CERAMIDE GALACTOSYLTRANSFERASE INHIBITORS FOR THE TREATMENT OF DISEASE
[FR] INHIBITEURS DE LA CÉRAMIDE GALACTOSYLTRANSFÉRASE POUR LE TRAITEMENT DE MALADIES摘要:本文描述了化合物、制备这种化合物的方法、含有这种化合物的药物组合物和药物,以及使用这种化合物治疗或预防与酶神经鞘糖脂转移酶(CGT)相关的疾病或紊乱的方法,例如溶酶体贮积症。溶酶体贮积症的例子包括 Krabbe 病和白质变性白血病。公开号:WO2018118838A1
文献信息
-
Water-Soluble and Recyclable Cyclopalladated Ferrocenylimine for Suzuki Coupling Reaction作者:Yang Li、Zhihua Fu、Lu Rao、Li Wang、Rui Li、Tiesheng Li、Yangjie WuDOI:10.1002/jccs.201300353日期:2014.3A series of new water‐soluble cyclopalladated ferrocenylimines were designed and prepared. They were efficient catalyst for Suzuki coupling reactions of aryl bromides and phenylboronic acid in neat water under ambient atmosphere. Among of these catalysts, the catalyst (C2D) could be reused for 6 times for the Suzuki coupling reaction of 4‐bromotoluene with phenylboronic acid in EtOH/H2O under ambient
-
4-Aminoquinoline derivatives申请人:John Wyeth & Brother Limited公开号:US03933829A1公开(公告)日:1976-01-20The disclosure describes new 4-aminoquinoline derivatives of general formula ##SPC1## And their acid addition salts, where X is a halogen atom or a trifluoromethyl group, Z is a hydrogen atom or a defined substituent, R is group of the formula --R.sub.3 N--A--NR.sub.1 R.sub.2 (II) --nr.sub.1 n--r.sub.3 (iii a) Or --N N NR.sub.1 R.sub.2 (III b) where A in formula II is a chain of 1 to 5 methylene groups which may be substituted with alkyl, the ring in formula IIIa and IIIb is a piperidine or pyrrolidine ring that may be substituted with alkyl and R.sub.1, R.sub.2 and R.sub.3 represent hydrogen or certain defined substituents. The new 4-aminoquinoline derivatives show anti-hypertensive activity and, in some cases, show one or more of the following activities: anti-malarial activity, anti-inflammatory activity, anti-trichomonal activity and inhibition of blood platelet aggregation. The present invention concerns new 4-aminoquinoline derivatives, a process for their preparation and pharmaceutical compositions containing them. The invention also concerns new intermediates useful for the preparation of the 4-aminoquinoline derivatives. The invention provides new 4-aminoquinoline derivatives of the general formula ##SPC2## And their acid addition salts, where I. X is a halogen atom or a trifluoromethyl group; Ii. Z is a hydrogen atom or a halogen atom, a trifluoromethyl group, a lower alkyl group, a lower alkoxy group, a hydroxyl group, a nitro group, an amino group or a mono- or di-alkyl substituted amino group, and Iii. R represents a group of the formula --NR.sub.3 --A--NR.sub.1 R.sub.2 (II) or ##SPC3## Wherein: A. in formula II, A represents a chain of 1 to 5 methylene groups, which may be substituted by one or more alkyl groups; B. in formula IIIa and IIIb the ring denotes a piperidine or pyrrolidine ring that may be substituted by one or more alkyl groups or by a divalent aliphatic chain substituting two different ring members of the piperidine or pyrrolidine ring; C. R.sub.1 represents a hydrogen atom, an alkyl group, an aralkyl group, an acyl group or an aryl group or, in formula II or IIIb, R.sub.1 and R.sub.2 may together form the diacyl residue of a dicarboxylic acid or R.sub.1 and R.sub.2 may together form a divalent radical such that R.sub.1 R.sub.2 NH is a secondary cyclic amine with 5 to 7 ring atoms; D. R.sub.2 is as defined above in connection with R.sub.1 or represents a hydrogen atom, an alkyl group, an aralkyl group or an acyl group; and E. R.sub.3 represents a hydrogen atom, a cycloalkyl group of 5 to 7 carbon atoms, an alkyl group, an aralkyl group, or an alkyl group substituted by a heterocyclic group, or an aliphatic chain joining the nitrogen atom member to another ring member of the ring in formula IIIa. The invention also provides a new class of compounds useful as intermediates for the preparation of compounds of formula I and their acid addition salts. These new compounds are benzenesulphonamides having the formula ##SPC4## and their acid addition salts, where R is as defined in connection with formula I, Z is as defined in connection with formula I or is a protected amino or hydroxyl group and Y is a hydrogen atom or a lower alkanoyl group. It will be apparent to those skilled in the art that the above definition of R includes moieties possessing an asymmetric carbon atom, especially for instance, in the cases where 1. A is linear chain of 1 to 15 methylene groups, the chain being monosubstituted by methyl or ethyl, or 2. R is of the formula V or VI ##SPC5## for example, in the cases where R denotes groups of the formula ##SPC6## where R.sub.1, R.sub.2 and R.sub.3 may be, for instance, hydrogen or lower alkyl. It is to be understood that general formulae I and IV are intended to encompass both enantiomers where the compound contains an asymmetric carbon atom and mixtures of the enantiomers, for instance, a racemic mixture of the enantiomers. General methods are recorded in the literature for the resolution of enantiomers. In the compounds of formula I, X preferably represents a halogen atom, for example, a chlorine or bromine atom, but may also represent a trifluoromethyl group. Illustrative meanings of Z in formulae I and IV include hydrogen, chlorine, bromine atoms and trifluoromethyl, lower alkyl or alkoxy (for example, methyl, ethyl, propyl, butyl, methoxy, ethoxy, propoxy and butoxy), hydroxyl, nitro, amino, methylamino, ethylamino, dimethylamino and diethylamino groups. Additionally in formula IV, Z may be protected amino or protected hydroxyl group, for example a group of the formula IX ##SPC7## In formulae II and IIIb R.sub.1 and R.sub.2 may be separate or may be joined together to form a divalent residue. The divalent residue is a diacyl residue of a dicarboxylic acid, for example, a group of the formula --CO--(CH.sub.2).sub.n --CO-- where n is 2 or 3, or ##SPC8## or is such that R.sub.1 R.sub.2 NH is a secondary cyclic amine with 5 to 7 ring atoms, for instance, piperidine, pyrrolidine or morpholine. R.sub.1, when in formula IIIa or when separate from R.sub.2 in formula II or IIIb, represents a hydrogen atom, an alkyl group, an aralkyl group, an acyl group or an aryl group, R.sub.2, when separate from R.sub.1 in formulae II and IIIb, represents a hydrogen atom, an alkyl group, an aralkyl group or an acyl group. R.sub.3 in formulae II and IIIa represents a hydrogen atom, a cycloalkyl group of 5 to 7 carbon atoms, an alkyl group, an aralkyl group, or an aliphatic chain joining the nitrogen ring atom to another ring atom of the ring shown in formula IIIa. Illustrative examples of such groups that can be denoted by R.sub.1, R.sub.2 or R.sub.3 will now be described. Alkyl groups are desirably lower alkyl groups, for example, methyl, ethyl, n- or i-propyl and n-butyl. Aryl groups particularly comprehend phenyl or phenyl substituted by one or more substituents. As substituents for a phenyl group there may be employed lower alkyl (for example, methyl, ethyl, propyl or butyl), lower alkoxy (for example, methoxy, ethoxy, propoxy or butoxy), nitro, halogen, (preferably chlorine or bromine), hydroxy, trifluoromethyl or amino (including mono- or dialkylamino, for instance, dimethylamino). Aralkyl groups are arylsubstituted alkyl groups, where the alkyl group is desirably a lower alkyl group (e.g. methyl, ethyl, propyl or butyl) and its aryl substituent may be phenyl or substituted phenyl, in which the one or more substituents for phenyl are as mentioned above. Acyl groups particularly include the acyl groups of the formula -- CO.R.sub.5 where R.sub.5 represents alkyl or aryl. As specific acyl groups there may be mentioned, for example, acetyl, propionyl, butanoyl, hexanoyl, benzoyl and benzoyl substituted by one or more of the above mentioned substituents for phenyl. As cycloalkyl of 5 to 7 carbon atoms there may be mentioned cyclopentyl, cyclohexyl and cycloheptyl. As alkyl substituted by a heterocyclyl group there may be mentioned lower alkyl such as methyl, ethyl, propyl or butyl, substituted by thienyl (for instance 2-thienyl), furyl, pyrrolyl, imidazolyl, pyrazolyl (for instance 4-pyrazolyl), indolyl, pyridyl (for instance 2- or 4-pyridyl), quinolyl, thiazolyl (specifically 2-, 4- or 5-thiazolyl), isothiazolyl or oxazolyl. As examples of A in formula II there may be mentioned methylene, dimethylene, trimethyleme, tetramethylene and pentamethylene and their mono- or di-(lower alkyl) substitution products, for example, groups of the formulae ##EQU1## The piperidine of pyrrolidine ring shown in formulae IIIa and IIIb may be substituted with one or more alkyl groups, preferably lower alkyl groups, for example, methyl, ethyl, propyl or butyl. As examples of R containing a piperidine or pyrrolidine ring there may be mentioned groups of the formula: ##SPC9## where m is 0 or 1; R.sub.1, R.sub.2 and R.sub.3 are as defined above; and R.sub.6, R.sub.7, R.sub.8 and R.sub.9, which may be the same or different may be hydrogen or lower alkyl, for instance, methyl, ethyl, propyl or butyl. In formula IV the symbol Y denotes a hydrogen atom or a lower alkanoyl group, for example, acetyl, propionyl, butanoyl or hexanoyl. In formulae I and IV the -- SO.sub.3 R group is preferably at the para-position relative to the 7-substituted-4-quinolylamino group in formula I and the group Y--NH-- in the case of formula IV. Thus the preferred new compounds of the invention are those of the formulae Ia and IVa ##SPC10## The term "lower" as used herein in connection with such groups as "alkanoyl", "alkyl" or "alkoxy" denotes that the group contains up to 6 carbon atoms, preferably up to 4 carbon atoms. Examples of acid addition salts are those formed from inorganic and organic acids and in particular include sulphate, hydrochloride, hydrobromide, hydroiodide, nitrate, phosphate, sulphonate (such as the methanesulphonate and p-toluene-sulphonate), acetate, maleate, fumarate, tartrate, malonate, citrate and formate. The compounds of the formula I may be made by building the compound up by known reactions. In particular the sulphonamide linkage shown in formula I may be formed by sulphonylation of an appropriate amine, and an amino-benzene sulphonamide may be converted to the secondary amine by introducing the 7-(halo or trifluoromethyl)-4-quinolyl group in known manner. The invention provides a method of making compounds of the formula I and their acid addition salts, wherein a compound of the formula RH, where R is as defined in connection with formula I, or, where necessary or desired, a corresponding compound with a protecting group, is sulphonylated to introduce the sulphonyl group of formula XIV ##SPC11## where X is defined in connection with formula I and Z is as defined in connection with formula IV. As sulphonating agent there may particularly be used a sulphonyl chloride of formula XV ##SPC12## where X is as defined above and Z is as defined in connection with formula IV. Alternatively, a compound of the formula XVI ##SPC13## (where R and Z are as defined in connection with formula IV is reacted with a compound of formula (XVII) ##SPC14## (where X is as defined above in connection with formula I and Q denotes a group or atom replaceable by nucleophilic attack by compound of formula XVI). Q is for example, an iodine atom, a bromine atom or a chloride atom or an organosulphonate group, for instance, p-toluenesulphonate. Where necessary or if desired, the process may also include removal of a protecting group, and if desired, conversion of a free base form of compound of formula I into an acid addition salt or conversion of an acid addition salt form of a compound of formula I into the corresponding free base form. Starting materials of formula RH and formulae XV are known compounds or, if new, are accessible by conventional methods. The sulphonylation method may be carried out by reacting the compound of formula XV with the compound of formula RH or a corresponding compound with a protecting group in chloroform in the presence of a saturated sodium carbonate solution. It will be apparent to those skilled in the art that certain unacylated compounds of formula RH may present more than one potentially reactive location for sulphonylation. Undesired sulphonylation may be avoided by chemical protection with removable blocking groups or other means. For example, the compounds of the formula ##SPC15## (wherein n is 0 or 1) may be sulphonylated at the ring nitrogen atom by using a starting material in which the NH.sub.2 function is protected with a blocking group which is removed after acylation. Compounds of formula I and their acid addition salts, in which, in formula IIIa, R.sub.3 denotes hydrogen, may be prepared by using, for example, a benzyl group as removable protecting group. Thus a starting compound of formula: ##SPC16## in which R.sub.3 is benzyl is sulphonylated and the protecting group is removed after sulphonylation by debenzylation. Debenzylation may be carried out using sodium in liquid ammonia or by catalytic hydrogenation under conditions such that the 7-halo- or 7-trifluoromethyl substituents on the quinolyl group is not removed. In addition, compounds of formula RH include compounds where a substituent on an aryl group or heterocyclyl group is susceptible to sulphonylation, e.g. a free hydroxyl or amino substituent. Such substituents may be protected with a removable blocking group which is cleaved off after sulphonylation. Sulphonylating derivatives for introducing the group of formula XIV include protection for a group Z sensitive to sulphonylation. For example, a final product in which Z is an amino function can be formed by using a sulphonylating derivative of the acid formula XX. ##SPC17## and, after sulphonylation, converting the phthalimido group to an NH.sub.2 group by reaction with hydrazine. The new compounds of the invention are comparatively stable to hydrolysis and therefore favour protecting groups that are readily hydrolysed off under acid or basic conditions. Compounds of the formula IV and their acid addition salts are accessible by a process wherein a compound formula RH where R is as defined in formula I, or a corresponding compound with a protecting group, is sulphonylated to introduce the sulphonyl group of formula: ##SPC18## where Y' is a lower alkanoyl group and Z is as defined above in connection with formula IV. The sulphonylating agent used is preferably the sulphonyl chloride of formula: ##SPC19## where Y' and Z are as defined in formula XXI. The corresponding sulphonylation product has the formula: ##SPC20## This product may be isolated as such or as an acid addition salt. This product may then be converted to the compound of formula XVI ##SPC21## by hydrolysis, preferably under alkaline conditions, to remove the lower alkanoyl group Y'. The compound of formula XVI may be recovered as the free base or as an acid addition salt. The reaction of the primary amine XVI with the compound of formula XVII may be carried out at elevated temperature in the presence of a suitable acidic solvent for example phenol or dilute hydrochloric acid. The reaction products may be recovered from the reaction mixtures by standard isolation procedures. In certain cases it is expedient to incorporate a protecting group for amino in the compound of formula XVI to reduce or preclude undesired reaction of the compounds of formula XVII with a primary or secondary amino function in the group R. In such cases the protecting group is removed after the reaction with the compound of formula XVII. The compounds of formula I may be isolated in free base form or as an acid addition salt. Acid addition salts may be converted into the free base in conventional manner. The free base forms may be converted into acid addition salts in conventional manner, for instance, by adding ethereal hydrogen chloride to a solution of the free base where a hydrochloride salt is desired. The sulphonylating agents used for reaction with a compound of formula RH can be prepared in known manner from the corresponding sulphonic acid. The sulphonic acid may be prepared by reacting an aminobenzene -sulphonic acid of the formula: ##SPC22## where Z is as defined in connection with formula IV with a compound of formula XVII ##SPC23## where X is a halogen atom or a trifluoromethyl group and Q is a group or atom replaceable by nucleophilic attack by the compound of formula XXIV. Q is for example, an iodine, bromine or chlorine atom. The secondary amine reaction product may be isolated as such or in the form of one of its salts. It may be purified salt formation and liberating the acid from the salt. The sulphonic acid or its salt may be converted into sulphonylating derivatives of the acid in known manner. For example the sulphonyl chloride of formula XV may be formed by reacting the corresponding sulphonic acid with thionyl chloride in the presence of dimethylformamide as catalyst. Some of the compounds of formula I may also be prepared by another method using the amide of the general formula XXV ##SPC24## where X is as defined above and Z is as defined in respect of formula IV. The amide can be prepared by reaction of the sulphonyl chloride of formula XV with ammonium hydroxide solution and isolated from the reaction mixture. The invention also provides a process for the preparation of a compound having general formula I wherein R is a group of formula II or IIIa and their acid addition salts. The compounds wherein R is a group of formula II and R.sub.3 is a hydrogen atom and wherein R is a group of formula IIIa whilst R.sub.1 is a hydrogen atom can be obtained by alkylation of the amide of formula XXV in aqueous or alcoholic alkaline solution. By means of this alkylation the group --A--NR.sub.1 R.sub.2 or the group ##SPC25## is introduced. The alkylating agent used is preferably the halide of formula: Hal--A--NR.sub.1 R.sub.2 (XXVI) ##SPC26## where Hal stands for a halogen atom preferably chlorine, bromine or iodine. Alternatively the corresponding sulphonates or organosulphonates may be used as alkylating agents. The compounds of formula I where R has formula II and R.sub.3 is an alkyl group, an aralkyl group or an alkyl group substituted by a heterocyclic group may be prepared by two successive alkylations of the amide of formula XXV, each carried out in aqueous or alcoholic alkaline solution. In this case the alkylation carried out to introduce the group R.sub.3 is preferably the second of the two reactions. Thus, for example, compounds of formula I where R is a group of formula II may be prepared by the reaction sequence ##SPC27## where Qu is the 7-(halo or trifluoromethyl)-4-quinolyl residue. Similarly the compounds of formula I where R is a group of formula IIIa and R.sub.1 is an alkyl or aralkyl group may be prepared by two successive alkylations of the amide of formula XXV in aqueous or alcoholic alkaline solution. One such alkylation introduces the group ##SPC28## whilst the other introduces the group R.sub.1. The reaction medium used for the alkylation of the amide of formula XXV may be an aqueous solution of sodium or potassium hydroxide. Alternatively the alkaline solution may be prepared from a lower alkanol, for example ethanol. The products of the alkylation steps may be recovered as such or as the acid addition salts in accordance with conventional isolation procedures. The compounds of formula I and their pharmaceutically acceptable acid addition salts are indicated for pharmacological usage and, in some cases, for use as intermediates for the preparation of other compounds of formula I. For instance, the compounds of formula I demonstrate anti-hypertensive activity and, in some cases, also demonstrate at least one of the following activities: anti-malarial activity, anti-inflammatory activity, anti-trichomonal activity and inhibition of blood platelet aggregation. For example, in addition to their anti-hypertensive activity, 4-(7-chloro-4-quinolyamino)-N-(1-ethyl-3-piperidyl)benzenesulphonamide shows anti-inflammatory activity, anti-trichomonal activity and inhibition of blood platelet aggregation and 4-dimethylamino-1-[4-(7-chloro-4-quinolyamino)-benzenesulphonyl]-piperdine shows anti-malarial and anti-trichomonal activity. The compounds are evaluated for their activity by testing in standard procedures. Some of the compounds of the invention may also be used as intermediates for the preparation of other compounds conforming with formula I. For example, compounds containing a phthalimido group as --NR.sub.1 R.sub.2 in formula II or IIb may be subjected to cleavage using hydrazizne to form a corresponding compound containing an amino group (--NH.sub.2) as --NR.sub.1 R.sub. 2 and compounds containing an amino group may be alkylated to form a corresponding compound with an alkyl-substituted amino groups. The invention also includes pharmaceutical compositions containing as active ingredients a compound of formula (I) or a pharmaceutically acceptable acid addition salt thereof, which may be micronised if desired. In addition to the active ingredient, said compositions also contain a non-toxic carrier. Any suitable carrier known in the art can be used to prepare the pharmaceutical compositions. In such a composition, the carrier may be a solid, liquid or mixture of a solid and a liquid. Solid form compositions include powders, tablets and capsules. A solid carrier can be one or more substances which may also act as flavoring agents, lubricants, solubilisers, suspending agents, binders, or tablet-disintegrating agents; it can also be an encapsulating material. In powders the carrier is a finely divided solid which is in admixture with the finely divided active ingredient. In tablets the active ingredient is mixed with a carrier having the necessary binding properties in suitable proportions and compacted in the shape and size desired. The powders and tablets preferably contain from 5 to 99, preferably 10-80% of the active ingredient. Suitable solid carriers are magnesium carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch gelatin, tragacanth, methyl cellulose, sodium carboxymethyl cellulose, a low melting wax, and cocoa butter. The term "composition" is intended to include the formulation of an active ingredient with encapsulating material as carrier to give a capsule in which the active ingredient (with or without other carriers) is surrounded by carrier, which is thus in association with it. Similarly cachets are included. Sterile liquid form compositions include sterile solutions, suspensions, emulsions, syrups and elixirs. The active ingredient can be dissolved or suspended in a pharmaceutically acceptable sterile liquid carrier, such as sterile water, sterile organic solvent or a mixture of both. Preferably a liquid carrier is one suitable for parenteral injection. Where the active ingredient is sufficiently soluble it can be dissolved in normal saline as a carrier; if it is too insoluble for this it can be dissolved in a suitable organic solvent, for instance aqueous propylene glycol or polyethylene glycol solutions. Aqueous propylene glycol containing from 10 to 75% of the glycol by weight is generally suitable. In other instances compositions can be made by dispersing the finely-divided active ingredient in aqueous starch or sodium carboxymethyl cellulose solution, or in a suitable oil, for instance arachis oil. Liquid pharmaceutical compositions which are sterile or suspensions can be utilised by intramuscular, intraperitoneal or subcutaneous injection. In many instances a compound is orally active and can be administered orally either in liquid or solid composition form. Preferably the pharmaceutical composition is in unit dosage form. In such form, the composition is sub-divided in unit doses containing appropriate quantities of the active ingredient; the unit dosage form can be a packaged composition, the package containing specific quantities of compositions, for example packeted powders or vials or ampoules. The unit dosage form can be a capsule, cachet or tablet itself, or it can be the appropriate number of any of these in package form. The quantity of active ingredient in a unit dose of composition may be varied or adjusted from 5 mg. or less to 500 or more, according to the particular need and the activity of the active ingredient. The invention also includes the compounds in the absence of carrier where the compounds are in unit dosage form.本发明涉及一类新的4-氨基喹啉衍生物,其通式为##SPC1##及其酸加成盐,其中X为卤素原子或三氟甲基,Z为氢原子或特定取代基,R为公式--R3N--A--NR1R2(II)、--NR1N--R3(IIIa)或--NNNR1R2(IIIb)的基团,其中公式II中的A为1至5个亚甲基链,该链可被烷基取代,公式IIIa和IIIb中的环为哌啶或吡咯烷环,该环可被烷基取代,R1、R2和R3代表氢或特定定义的取代基。这些新的4-氨基喹啉衍生物显示出抗高血压活性,并且在某些情况下,显示出以下一种或多种活性:抗疟疾活性、抗炎活性、抗毛滴虫活性和血小板聚集抑制作用。本发明涉及新的4-氨基喹啉衍生物、其制备方法以及含有它们的药物组合物。本发明还涉及用于制备4-氨基喹啉衍生物的有用的新中间体。 本发明提供了一类新的4-氨基喹啉衍生物,其通式为##SPC2##及其酸加成盐,其中:I. X为卤素原子或三氟甲基;II. Z为氢原子或卤素原子、三氟甲基、低级烷基、低级烷氧基、羟基、硝基、氨基或单或双烷基取代的氨基;III. R代表公式--NR3--A--NR1R2(II)或##SPC3##的基团,其中:A. 在公式II中,A代表1至5个亚甲基链,该链可被一个或多个烷基取代;B. 在公式IIIa和IIIb中,环表示哌啶或吡咯烷环,该环可被一个或多个烷基取代,或被取代两个不同环成员的二价脂族链取代;C. R1代表氢原子、烷基、芳烷基、酰基或芳基,或者在公式II或IIIb中,R1和R2可以一起形成二羧酸的二酰基残基,或者R1和R2可以一起形成二价基团,使得R1R2NH是具有5至7个环原子的二级环胺;D. R2如上文关于R1所定义,或代表氢原子、烷基、芳烷基或酰基;E. R3代表氢原子、5至7个碳原子的环烷基、烷基、芳烷基,或被杂环基团取代的烷基,或连接氮原子成员与公式IIIa中环的另一环原子的脂族链。 本发明还提供了一类新的化合物,作为制备公式I化合物及其酸加成盐的有用中间体。这些新化合物是具有公式##SPC4##的苯磺酰胺及其酸加成盐,其中R如公式I中所定义,Z如公式I中所定义或为保护的氨基或羟基,Y为氢原子或低级烷酰基。 本发明还包括制备公式I化合物及其酸加成盐的方法,其中化合物RH(其中R如公式I中所定义)或必要时或希望时的相应化合物与保护基团,被磺酰化以引入公式XIV的磺酰基团##SPC11##其中X如公式I中所定义,Z如公式IV中所定义。 本发明还提供了制备公式IV化合物及其酸加成盐的方法,其中化合物RH(其中R如公式I中所定义)或必要时或希望时的相应化合物与保护基团,被磺酰化以引入公式##SPC18##的磺酰基团,其中Y'为低级烷酰基,Z如上文关于公式IV所定义。 本发明的化合物显示出抗高血压活性,并且在某些情况下,还显示出抗疟疾活性、抗炎活性、抗毛滴虫活性和血小板聚集抑制作用。本发明还包括含有作为活性成分的公式I化合物或其药学上可接受的酸加成盐的药物组合物。这些组合物可以以固体形式(如粉末、片剂和胶囊)或液体形式(如无菌溶液、悬浮液、乳剂、糖浆和酏剂)存在,并且可以包含非毒性载体。本发明还包括以单位剂量形式存在的化合物。
-
Synthesis and characterization of bis(diphenylphosphinomethyl)amino ligands and their Ni(II), Pd(II) complexes: Application to hydrogenation of styrene作者:Mustafa Keleş、Orhan Altan、Osman SerindaǧDOI:10.1002/hc.20384日期:——Transition metal complexes of Ni(II) and Pd(II) with ditertiary bis(diphenylphosphinomethyl)amino ligands, (Ph2PCH2)2NC(CH3)3 (1) and [(Ph2PCH2)Nm-PhSO3]Na (2) (Ph = phenyl), have been synthesized in good yields under nitrogen atmosphere by Schlenk technique. All complexes have been characterized using elemental analyses and spectroscopic techniques such as atomic absorption, FT-IR, and NMR (1H, 31P)Ni(II) 和 Pd(II) 与二叔双 (二苯基膦甲基) 氨基配体 (Ph2PCH2)2NC(CH3)3 (1) 和 [(Ph2PCH2)Nm-PhSO3]Na (2) (Ph = 苯基) 的过渡金属配合物),已通过 Schlenk 技术在氮气氛下以良好的收率合成。所有配合物均已使用元素分析和光谱技术(例如原子吸收、FT-IR 和 NMR(1H,31P))进行表征。原子吸收光谱分析表明配体1和2与NiCl2·6H2O和[PdCl2(COD)]的反应以1:1的摩尔比发生。发现金属配合物 [NiCl2(Ph2PCH2)2Nm-PhSO3Na] (4) 和 [PdCl2(Ph2PCH2)2Nm-PhSO3Na] (6) 可溶于乙醇和水溶液,也微溶于水。通过循环伏安法研究了配合物的电化学行为。在苯乙烯氢化成乙苯的整个过程中测试了 Pd(II) 配合物的催化作用。© 2008 Wiley Periodicals
-
Silicon Nanowire Arrays - A New Catalyst for the Reduction of Nitrobenzene Derivatives作者:Yajun Xu、Lei Wang、Wenwen Jiang、Hongwei Wang、Jianlin Yao、Qinghua Guo、Lin Yuan、Hong ChenDOI:10.1002/cctc.201300480日期:2013.12Silicon nanowire arrays (SiNWAs) are under extensive investigation for solar cells and biomedical applications. This study reports for the first time that hydrogen fluoride‐treated (H)‐SiNWAs are an efficient catalyst for the reduction of nitrobenzene derivatives. We show that SiNWAs, after hydrogen fluoride treatment, have a high catalytic activity in p‐nitrophenol (PNP) reduction. The conversion硅纳米线阵列(SiNWA)正在针对太阳能电池和生物医学应用进行广泛研究。这项研究首次报道了氟化氢处理(H)-SiNWAs是还原硝基苯衍生物的有效催化剂。我们表明,氟化氢处理后的SiNWAs在对硝基苯酚(PNP)还原中具有很高的催化活性。H-SiNWAs对PNP的转化率随时间增加,并且在30分钟内几乎完成,因此表明其催化活性与铂纳米颗粒相当。SiNWAs的催化活性与表面的化学组成和特定形态密切相关。硅表面上的H键对于活性至关重要,具有较长纳米线的阵列显示出较高的催化活性。而且,可以通过氟化氢处理容易地再生活性。人们还发现,H-SiNWAs表现出对其他硝基苯衍生物的还原相似的催化活性,如p硝基苯胺和钠米-nitrobenzenesulfonate。结论是,H-SiNWAs可以被认为是用于还原硝基苯衍生物的贵金属催化剂的环保替代品。
-
Thermal oxidation to regenerate sulfone poisoned Pd-based catalyst: effect of the valence of sulfur作者:Tieyong Xu、Qunfeng Zhang、Dahao Jiang、Qiuxia Liang、Chunshan Lu、Jie Cen、Xiaonian LiDOI:10.1039/c4ra03546a日期:——Sulfur deactivation is a serious problem which largely limits the industrial application of noble metals as catalysts. Here we report a thermal oxidation method to regenerate sulfone poisoned Pd/C catalyst applied in the hydrogenation of sodium-m-nitrobenzene sulfonate (SNS). It was found that the initial activity of Pd/C catalyst could be substantially recovered after treating it in air at temperatures as low as 100 °C. And the catalyst could be reused for at least 20 times without the significant loss of activity. The properties of deactivated and regenerated catalysts were studied in detail by BET measurement, X-ray photoelectron spectroscopy (XPS), temperature programmed desorption (TPD), and Fourier transform infrared spectroscopy (FT-IR). The results indicated that the main surface sulfur species found on deactivated and regenerated Pd surfaces were Sn and sulfate (SO4), respectively. The change of the valence of sulfur species was found to be the key factor influencing the catalytic activity of the Pd-based catalyst.
表征谱图
-
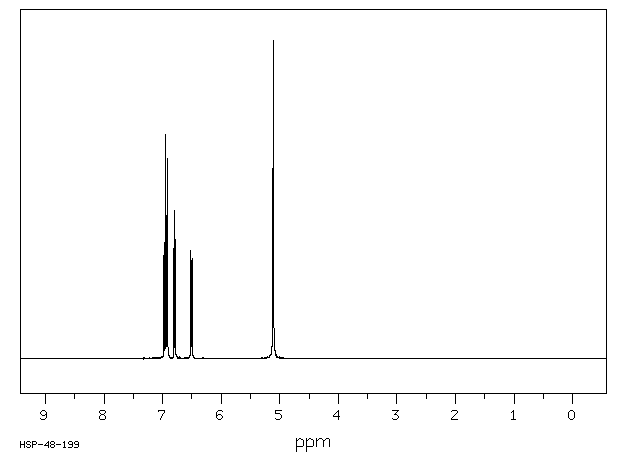
氢谱1HNMR
-
质谱MS
-
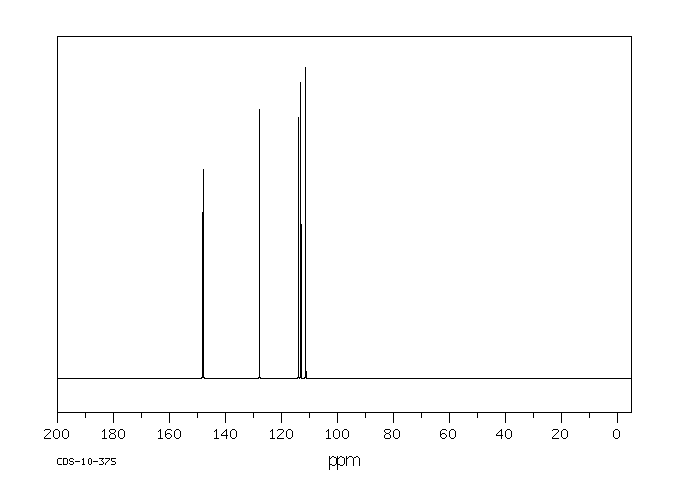
碳谱13CNMR
-
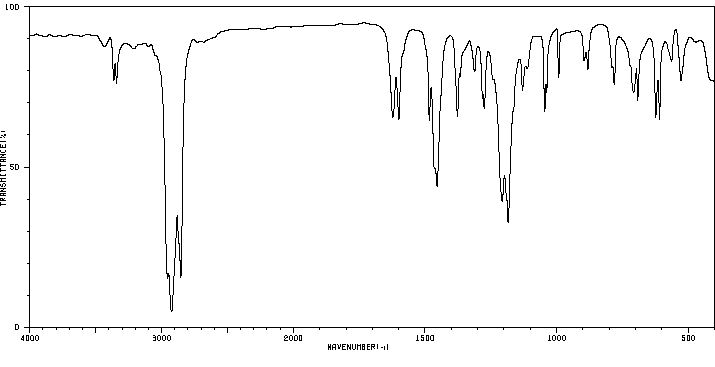
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫