5-十一炔 | 2294-72-6
中文名称
5-十一炔
中文别名
——
英文名称
undec-5-yne
英文别名
5-undecyne
CAS
2294-72-6
化学式
C11H20
mdl
MFCD00041657
分子量
152.28
InChiKey
VRQLDBSWBBKOCR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-74.1°C
-
沸点:78°C 10mm
-
密度:0,775 g/cm3
-
保留指数:1127
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.818
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S16
-
危险类别码:R10
-
海关编码:2901299090
-
危险品运输编号:1993
SDS
5-Undecyne Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 5-Undecyne
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Combustible liquid
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wear protective gloves and eye/face protection.
IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
[Response]
induce vomiting.
Store in a well-ventilated place. Keep cool.
[Storage]
Store locked up.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 5-Undecyne
Percent: ....
CAS Number: 2294-72-6
Chemical Formula: C11H20
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
5-Undecyne
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
Respiratory protection:
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
5-Undecyne
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 197°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.77
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Open flame
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
5-Undecyne
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 5-Undecyne
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Combustible liquid
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wear protective gloves and eye/face protection.
IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
[Response]
induce vomiting.
Store in a well-ventilated place. Keep cool.
[Storage]
Store locked up.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 5-Undecyne
Percent: ....
CAS Number: 2294-72-6
Chemical Formula: C11H20
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
5-Undecyne
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
Respiratory protection:
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
5-Undecyne
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 197°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.77
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Open flame
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
5-Undecyne
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:A new route to α-trialkylsilyl aldehydes. The first isolation of α-trimethylsilyl aldehydes摘要:通过溴锂交换和三烷基硅基的 1â3 迁移,从三甲基硅基和三乙基硅基 δ溴烯醇醚 1 中得到δ-三甲基硅基和δ-三乙基硅基醛 6。DOI:10.1039/c39930001763
-
作为产物:描述:1,5-二碘戊烷 在 正丁基锂 、 nickel(II) salen 、 tetramethylammonium tetrafluoroborate 作用下, 以 四氢呋喃 、 正己烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 0.5h, 生成 5-十一炔参考文献:名称:二甲基甲酰胺中碳阴极上电生成的镍(I)Salen存在下卤代炔烃的催化还原和分子内环化摘要:通过[[2,2 ' -[1,2-乙二基双(亚硝甲基))]双[苯酚基]催化还原1-碘-或1-溴-5-癸炔可以方便地制备亚萘基环戊烷,产率高达86%。] -N,N ',O,O '在包含四氟硼酸四甲基铵的二甲基甲酰胺中的碳阴极上电生成的]镍酸酯(I)。该电合成可以在卤代炔是电惰性的电势下完成,并且可以在室温下30分钟内完成。尝试在类似条件下分别由1-卤代4-壬炔和11-卤代5-十一炔合成戊二烯环丁烷和戊二烯环己烷可提供非常低的收率(分别为2%和6%)。衍生自各种卤代炔烃的其他产物是二聚体,炔烃和1-炔烃。由1-卤代4-壬炔和11-卤代5-十一炔产生的二聚体(alkadiynes)的产率为80%至89%,而icosa-5,15-diyne(由1-卤代获得的二聚体)发现-5-decyne)的收率明显较低(≤13%)。炔烃和1-炔烃的产率分别为3-10%和2-3%。提出了一种机制方案,该方案涉及由镍(DOI:10.1021/jo052007a
文献信息
-
The reaction of lithium trialkylalkynylborate with methanesulphinyl chloride作者:M. Naruse、K. Utimoto、H. NozakiDOI:10.1016/s0040-4020(01)97353-3日期:1974.1Treatment of lithium trialkylalkynylborates (1) with methanesulphinyl chloride gives internal acetylenes (2) in good yields. The reaction proceeds via β-methanesulphinylvinylboranes 3, followed by cis elimination of methanesulphinyl group and dialkylboron groups. The reaction mixture of B-alkyl-9-BBN and 1-lithio-1-heptyne has been treated with methanesulphinyl chloride to provide mainly a cyclooctane
-
Iridium-Catalyzed Carbonylative Synthesis of Halogen-Containing Quinolin-2(1<i>H</i>)-ones from Internal Alkynes and Simple Anilines作者:Fengxiang Zhu、Yahui Li、Zechao Wang、Xiao-Feng WuDOI:10.1002/adsc.201600680日期:2016.11.3straightforward synthesis of halogen‐containing quinolin‐2(1H)‐ones. The reaction proceeds without preactivation and directing groups through direct N–H and C–H bond activation with a broad substrate scope and high efficiency. Halogen functional groups can be well tolerated here. Remarkably, this is the first example of an iridium‐catalyzed carbonylative C–H activation of anilines.
-
Stereoselective Synthesis ofanti-1,4-Diols by a BH3⋅THF-Mediated Rearrangement of 1,2-Disubstituted Cyclobutenes作者:Kolja M. Knapp、Bernd Goldfuss、Paul KnochelDOI:10.1002/chem.200304902日期:2003.11.7A new stereoselective rearrangement of cyclobutylboranes, obtained by the hydroboration of 1,2-disubstituted cyclobutenes, provides anti-1,4-diols with good-to-excellent diastereoselectivity. The mechanism of the rearrangement is discussed based on theoretical calculations.
-
A new general synthesis of aliphatic and terminal alkynes: flash vacuum pyrolysis of β-oxoalkylidenetriphenylphosphoranes作者:R. Alan Aitken、J. Ian AthertonDOI:10.1039/c39850001140日期:——conditions the thermal elimination of Ph3PO from β-oxoalkylidenetriphenylphosphoranes, previously confined to cases with an α-electron withdrawing group, has been extended to provide a general, high yielding synthesis of aliphatic and terminal alkynes.通过使用快速真空条件,从以前局限于具有电子吸电子基团的情况下,从β-氧代亚烷基三苯并三苯膦中消除Ph 3 PO的范围扩大了,从而提供了一种通常的高产率的脂肪族和末端炔烃合成方法。
-
Copper-Catalyzed Cross-Coupling of Alkyl and Aryl Grignard Reagents with Alkynyl Halides作者:Gérard Cahiez、Olivier Gager、Julien BuendiaDOI:10.1002/anie.200905816日期:——Good old copper! A new general procedure to couple aliphatic and aromatic Grignard reagents with alkynyl halides under copper catalysis is described (see scheme; NMP=N‐methylpyrrolidinone). The reaction is chemoselective and allows preparation of a vast array of simple and functionalized internal alkynes in high yields.
表征谱图
-
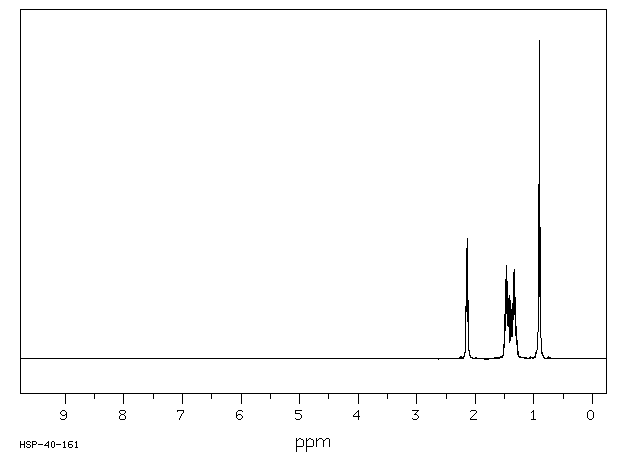
氢谱1HNMR
-
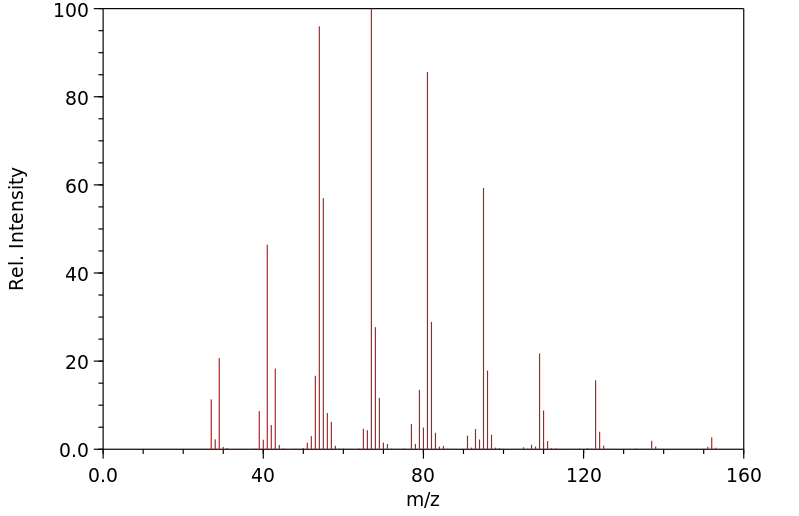
质谱MS
-
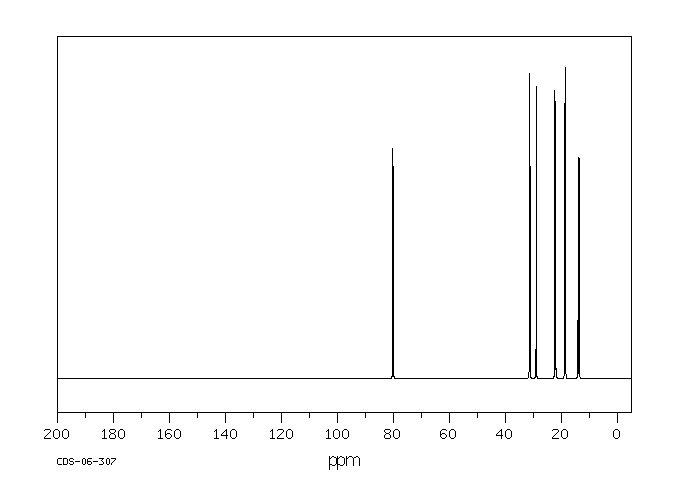
碳谱13CNMR
-
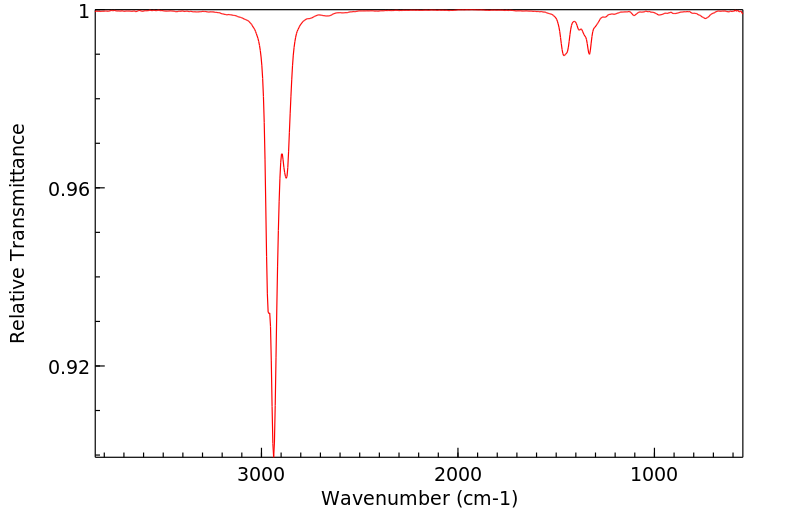
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-