2,6-二甲氧基吡啶 | 6231-18-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:178-180 °C (lit.)
-
密度:1.053 g/mL at 25 °C (lit.)
-
闪点:143 °F
-
保留指数:1028.1
-
稳定性/保质期:
常温常压下稳定,为液体。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:31.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:3.2
-
危险品标志:Xn
-
安全说明:S26,S36/37/39,S39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:1993
-
危险类别:3.2
-
包装等级:III
-
危险标志:GHS05,GHS07
-
危险性描述:H302,H315,H318,H335
-
危险性防范说明:P261,P280,P305 + P351 + P338
-
储存条件:避光,存放在阴凉干燥处,并密封保存。
SDS
模块 1. 化学品
产品名称: 2,6-Dimethoxypyridine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
急性毒性(经口) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
2,6-二甲氧基吡啶 修改号码:5
模块 2. 危险性概述
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,6-二甲氧基吡啶
百分比: >97.0%(GC)
CAS编码: 6231-18-1
分子式:
C7H9NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
2,6-二甲氧基吡啶 修改号码:5
模块 8. 接触控制和个体防护
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 179 °C
闪点: 61°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.09
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
2,6-二甲氧基吡啶 修改号码:5
模块 12. 生态学信息
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
2,6-二甲氧基吡啶广泛应用于多种药物分子中,包括酶抑制剂/激动剂、抗艾滋药物、抗菌药和麻醉剂等。
制备将乙酸钯(Pd(OAc)₂)(0.023 mmol,1 mol%)、双吡唑基膦配体(0.047 mmol,2 mol%)加入到干燥的压力管中(10 mL)。随后向反应混合物中依次加入碳酸铯(Cs₂CO₃)(3.50 mmol,1.5 当量)、甲苯(2 mL)、2,6-二溴吡啶(2.34 mmol)和甲醇(2 mL)。将反应体系在80℃下搅拌过夜(24小时),然后冷却至室温。用乙酸乙酯(EtOAc,5 mL)稀释反应混合物,并通过硅藻土垫过滤。浓缩粗产物后,采用快速色谱法纯化,最终得到2,6-二甲氧基吡啶。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,6-dimethoxypyridine N-oxide 7259-54-3 C7H9NO3 155.153 2,6-二羟基吡啶 2-hydroxy-6-pyridone 626-06-2 C5H5NO2 111.1 3-羟基-2,6-二甲氧基吡啶 2,6-dimethoxypyridin-3-ol 885963-28-0 C7H9NO3 155.153 —— 3-iodo-2,6-dimethoxypyridine 214360-56-2 C7H8INO2 265.051 —— 3-deutero-2,6-dimethoxypyridine 1356156-14-3 C7H9NO2 140.146 3-溴-2,6-二甲氧基吡啶 3-bromo-2,6-dimethoxypyridine 13445-16-4 C7H8BrNO2 218.05 3-氨基-2,6-二甲氧基吡啶 2,6-dimethoxy-3-aminopyridine 28020-37-3 C7H10N2O2 154.169 3,5-二溴-2,6-二甲氧基吡啶 3,5-dibromo-2,6-dimethoxypyridine 16727-44-9 C7H7Br2NO2 296.946
反应信息
-
作为反应物:描述:参考文献:名称:Process for preparation of polyhydric alcohols摘要:根据该发明,制备多羟基醇的方法包括将具有受保护羟基的多羟基醇化合物置于存在具有25°C下酸解离指数(pKa)为-8至3的碱性化合物或酸性化合物的微波辐射中,以去除多羟基醇化合物的羟基的保护基。该发明可以通过轻松去除多羟基醇化合物中受保护羟基的保护基,提供一个工业上有利的制备多羟基醇的方法。公开号:US20020157939A1
-
作为产物:描述:参考文献:名称:一些卤代吡啶与甲醇盐和甲硫醇盐离子在二甲基甲酰胺中的反应摘要:2,6-和2,5-二溴吡啶和2,3-和3,5-二氯吡啶与异丙硫醇钠和甲硫醇钠的反应根据实验条件提供单取代或双取代的产物。相同的吡啶与甲醇钠反应,得到良好收率的单取代产物。仅在2,6-二溴-和3,5-二氯吡啶中容易发生双取代。还描述了从卤代甲氧基吡啶或卤代硫代甲氧基吡啶开始的一些甲氧基硫代甲氧基吡啶的合成。双(烷硫基)吡啶可被HMPA中的钠裂解,得到双(巯基)吡啶。DOI:10.1016/s0040-4020(01)96539-1
-
作为试剂:描述:5-(4-methoxyphenyl)hex-4-en-1-ol 在 2,6-二甲氧基吡啶 、 9-mesityl-10-methylacridinium tetrafluoroborate 、 4-甲苯硫酚 作用下, 以 1,2-二氯乙烷 为溶剂, 生成 (±)-(R,S)-2-(1-(4-methoxyphenyl)ethyl)tetrahydrofuran 、 2-(1-(4-methoxyphenyl)ethyl)tetrahydrofuran参考文献:名称:重新利用可见光激发烯还原酶进行非对映和对映选择性内酯合成摘要:已经开发出双重生物/光催化系统,用于实现以前难以实现的非对映选择性和对映选择性自由基内酯化。通过整合定向进化和光诱导单电子氧化,我们重新利用了工程烯还原酶来引导非天然自由基C−O形成,从而提供了多种具有邻位立构中心的对映增强型5元和6元内酯,其中部分带有季铵基团立体中心。DOI:10.1002/anie.202402673
文献信息
-
Selective C–H Iodination of (Hetero)arenes作者:Lalita Tanwar、Jonas Börgel、Johannes Lehmann、Tobias RitterDOI:10.1021/acs.orglett.1c01530日期:2021.7.2Iodoarenes are versatile intermediates and common synthetic targets in organic synthesis. Here, we present a strategy for selective C–H iodination of (hetero)arenes with a broad functional group tolerance. We demonstrate the utility and differentiation to other iodination methods of supposed sulfonyl hypoiodites for a set of carboarenes and heteroarenes.
-
Role of Mono-N-protected Amino Acid Ligands in Palladium(II)-Catalyzed Dehydrogenative Heck Reactions of Electron-Deficient (Hetero)arenes: Experimental and Computational Studies作者:Xuefeng Cong、Huarong Tang、Chao Wu、Xiaoming ZengDOI:10.1021/om400890p日期:2013.11.11We report here that mono-N-protected amino acids (MPAAs), an important environmentally compatible structural motif, enable acceleration of Pd(II)-catalyzed dehydrogenative Heck reactions between pyridines and electron-deficient arenes with simple alkenes, leading to diversely functionalized C3- or meta-selective alkenylated pyridines and benzenes via non-chelate-assisted C–H activation. A comprehensive我们在这里报告,单N保护的氨基酸(MPAAs),一种重要的环境相容性结构基序,能够加速Pd(II)催化的吡啶和电子不足的芳烃与简单烯烃之间的脱氢Heck反应,从而导致功能化的C3 -或meta通过非螯合物辅助的C–H活化选择性烯基化吡啶和苯。通过DFT计算进行的全面理论研究表明,MPAA配体的氨基支架通过最初的N–H活化容易地转化为X型配体,从而导致吡啶C–H裂解的活化障碍相对较低。然后,氨基的性质从X型配体转变为L型配体使得烯烃取代可以顺利进行,而羧基则可以形成分子内氢键,从而大大降低了碳pal触动的激活障碍。计算结果和动力学同位素效应测量结果支持了限速C–H活化,其机制涉及协同的金属化/去质子化途径,吸热值为31。
-
Multinuclear NMR spectra of [Pt(L)Cl<sub>3</sub>]<sup>−</sup> (L = pyridine derivatives) complexes and crystal structure of <i>trans</i>-Pt(2,6-di(hydroxymethyl)pyridine)<sub>2</sub>Cl<sub>2•</sub>2H<sub>2</sub>O作者:Fernande D. ,、Corinne Bensimon、André L. BeauchampDOI:10.1139/v96-241日期:1996.11.1observed at lower fields, except for complexes containing hydroxy or amine groups. The latter compounds were observed at higher fields, close to the signals of the Pt-unsubstituted pyridine compound. These results were explained in terms of the solvent effect. The chemical shifts δ(C) and the coupling constants J(13C–195Pt) were measured and the results interpreted with a view of obtaining information on[Pt(L)Cl3]-(L = 吡啶衍生物)类型的配合物被合成并通过 13C 和 195Pt NMR 光谱进行研究。在 -1720 和 -1897 ppm 之间观察到 195Pt 信号。未发现质子化吡啶衍生物的 δ(Pt) 和 pKa 之间存在相关性。化学位移随吡啶配体上的取代基而变化。除了含有羟基或胺基团的配合物外,在低场观察到具有邻位取代基的化合物。后一种化合物在更高的场中观察到,接近于 Pt 未取代的吡啶化合物的信号。这些结果用溶剂效应来解释。测量了化学位移 δ(C) 和耦合常数 J(13C–195Pt),并根据获得有关 Pt-N 键性质的信息来解释结果。检查了铂和吡啶配体之间 π 键合的可能性。吡啶环相对于铂平面的构象和...的能量
-
Synthetic Studies to Help Elucidate the Metabolism of the Preclinical Candidate TBAJ-876—A Less Toxic and More Potent Analogue of Bedaquiline作者:Peter J. Choi、Daniel Conole、Hamish S. Sutherland、Adrian Blaser、Amy S.T. Tong、Christopher B. Cooper、Anna M. Upton、Brian D. Palmer、William A. DennyDOI:10.3390/molecules25061423日期:——
Bedaquiline is a novel drug approved in 2012 by the FDA for treatment of drug-resistant tuberculosis (TB). Although it shows high efficacy towards drug-resistant forms of TB, its use has been limited by the potential for significant side effects. In particular, bedaquiline is a very lipophilic compound with an associated long terminal half-life and shows potent inhibition of the cardiac potassium hERG channel, resulting in QTc interval prolongation in humans that may result in cardiac arrhythmia. To address these issues, we carried out a drug discovery programme to develop an improved second generation analogue of bedaquiline. From this medicinal chemistry program, a candidate (TBAJ-876) has been selected to undergo further preclinical evaluation. During this evaluation, three major metabolites arising from TBAJ-876 were observed in several preclinical animal models. We report here our synthetic efforts to unequivocally structurally characterize these three metabolites through their independent directed synthesis.
贝达昆林是一种新型药物,2012年获得FDA批准用于治疗耐药结核病(TB)。尽管它对耐药结核病表现出高效性,但由于潜在的严重副作用,其使用受到限制。特别是,贝达昆林是一种非常亲脂性的化合物,具有长的终末半衰期,并且对心脏钾通道hERG表现出强效抑制作用,导致人体QTc间期延长,可能导致心律失常。为了解决这些问题,我们进行了一项药物发现计划,以开发改进的第二代贝达昆林类似物。从这个药物化学计划中,已选择了一个候选药物(TBAJ-876)进行进一步的临床前评估。在这个评估过程中,在几种临床前动物模型中观察到了TBAJ-876产生的三种主要代谢物。我们在这里报告了我们的合成努力,通过它们的独立定向合成来明确结构表征这三种代谢物。 -
COMPOUND COMPRISING PHENYL PYRIDINE UNITS申请人:Ye Qing公开号:US20090289547A1公开(公告)日:2009-11-26Organic compounds of formula I may be used in optoelectronic devices wherein R 1 is, independently at each occurrence, a C 1 -C 20 aliphatic radical, a C 3 -C 20 aromatic radical, or a C 3 -C 20 cycloaliphatic radical; R2 is, independently at each occurrence, a C 1 -C 20 aliphatic radical, a C 3 -C 20 aromatic radical, or a C 3 -C 20 cycloaliphatic radical; a is, independently at each occurrence, an integer ranging from 0-4; b is, independently at each occurrence, an integer ranging from 0-3; Ar 1 is a direct bond or heteroaryl, aryl, or alkyl or cycloalkyl; Ar 2 is heteroaryl, aryl, or alkyl or cycloalkyl; c is 0, 1 or 2; and n is an integer ranging from 2-4.化学式I的有机化合物可用于光电子器件,其中R1在每次出现时独立地是C1-C20脂肪基、C3-C20芳香基或C3-C20环脂肪基;R2在每次出现时独立地是C1-C20脂肪基、C3-C20芳香基或C3-C20环脂肪基;a在每次出现时独立地是0-4之间的整数;b在每次出现时独立地是0-3之间的整数;Ar1是直接键或杂环芳基、芳基、烷基或环烷基;Ar2是杂环芳基、芳基、烷基或环烷基;c为0、1或2;n是2-4之间的整数。
表征谱图
-
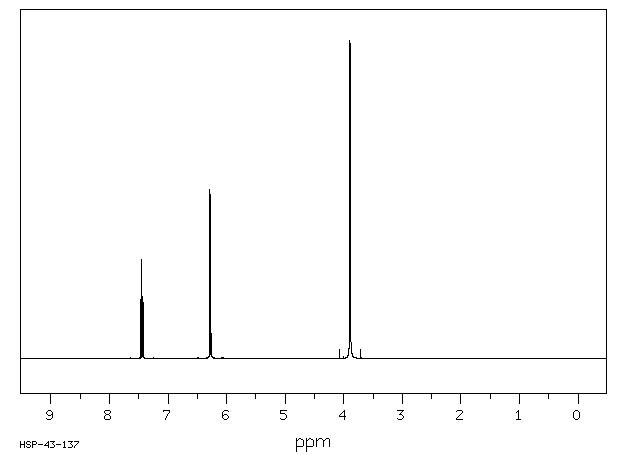
氢谱1HNMR
-
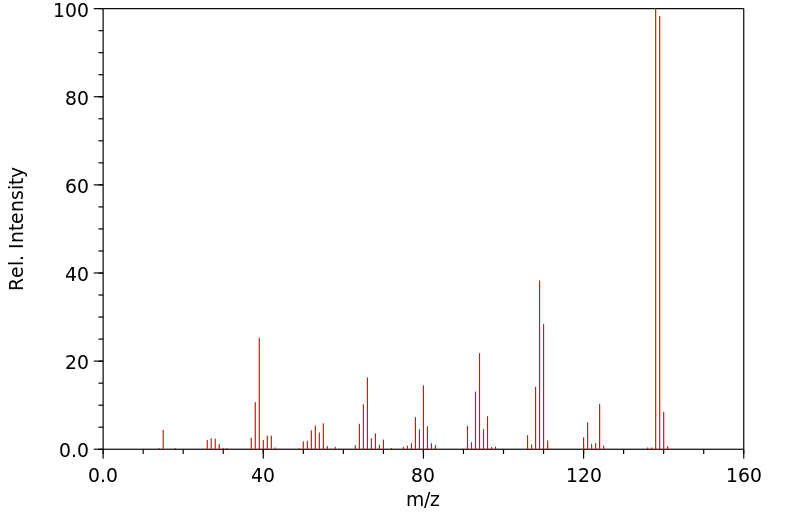
质谱MS
-
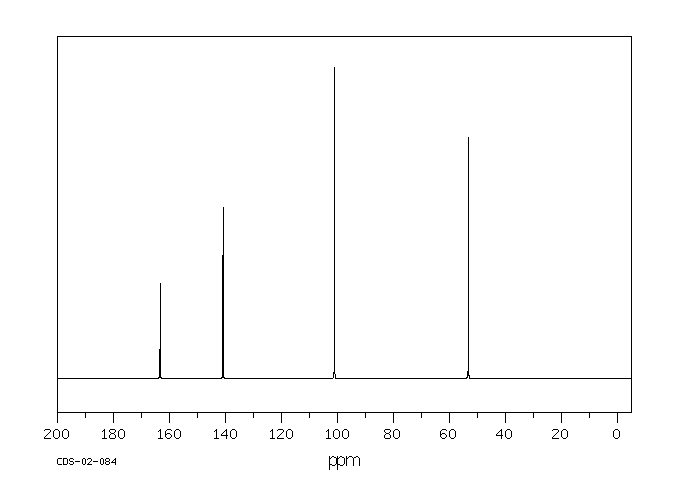
碳谱13CNMR
-
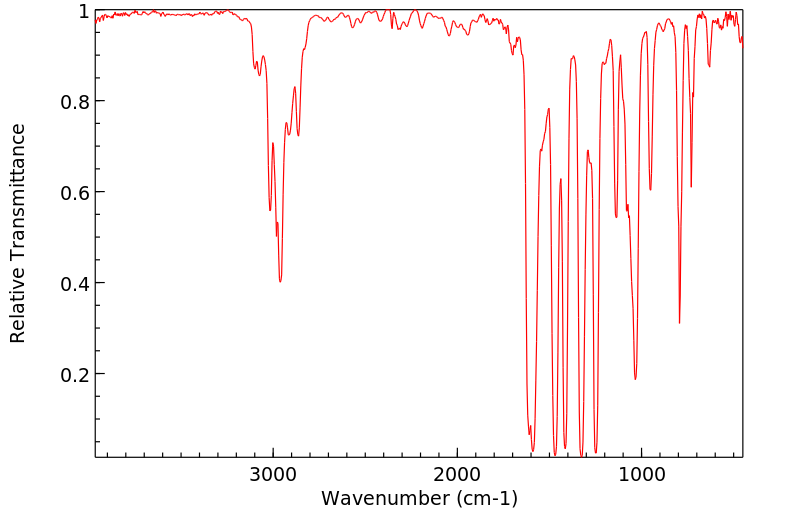
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息