2'-氯代联苯乙酮 | 635-84-7
中文名称
2'-氯代联苯乙酮
中文别名
2-氯-4'-苯基苯乙酮;2’-氯代联苯乙酮;2"-氯代联苯乙酮;2-氯-4"-苯基苯乙酮
英文名称
4-(chloroacetyl)biphenyl
英文别名
1-biphenyl-4-yl-2-chloroethanone;p-phenylphenacyl chloride;1-([1,1'-biphenyl]-4-yl)-2-chloroethan-1-one;2-chloro-4'-phenylacetophenone;2-chloro-1-(4-phenylphenyl)ethanone
CAS
635-84-7
化学式
C14H11ClO
mdl
——
分子量
230.694
InChiKey
IQEIGQFNDLINOT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:126°C
-
沸点:366.1±17.0 °C(Predicted)
-
密度:1.167±0.06 g/cm3(Predicted)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
安全说明:S26,S36/37/39
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。储存地点须远离氧化剂。
SDS
2-Chloro-4'-phenylacetophenone Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 2-Chloro-4'-phenylacetophenone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 2-Chloro-4'-phenylacetophenone
Percent: >99.0%(T)
CAS Number: 635-84-7
Synonyms: 4-Chloroacetylbiphenyl , 4-Phenylphenacyl Chloride
Chemical Formula: C14H11ClO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2-Chloro-4'-phenylacetophenone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale yellow
Odor: No data available
pH: No data available
2-Chloro-4'-phenylacetophenone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:126 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
2-Chloro-4'-phenylacetophenone
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 2-Chloro-4'-phenylacetophenone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 2-Chloro-4'-phenylacetophenone
Percent: >99.0%(T)
CAS Number: 635-84-7
Synonyms: 4-Chloroacetylbiphenyl , 4-Phenylphenacyl Chloride
Chemical Formula: C14H11ClO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2-Chloro-4'-phenylacetophenone
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale yellow
Odor: No data available
pH: No data available
2-Chloro-4'-phenylacetophenone
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:126 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
2-Chloro-4'-phenylacetophenone
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1'-biphenyl-4,4'-diylbis(2-chloroethanone) 24860-53-5 C16H12Cl2O2 307.176 联苯单乙酮 4-Acetylbiphenyl 92-91-1 C14H12O 196.249 —— 1-(biphenyl-4-yl)-2-chloro-2-(hydroxyimino)ethan-1-one 5337-28-0 C14H10ClNO2 259.692 —— 2-biphenyl-4-yl-glyoxylohydroximoyl chloride 5337-28-0 C14H10ClNO2 259.692 4-苯基苯甲酰基乙腈 3-([1,1'-biphenyl]-4-yl)-3-oxopropanenitrile 78443-35-3 C15H11NO 221.258 —— 2-chloro-1-(biphenyl-4-yl)ethanol 19177-51-6 C14H13ClO 232.71
反应信息
-
作为反应物:描述:参考文献:名称:通过氰化促进供体-受体共轭微孔聚合物中的电荷分离,用于氯化物的光催化还原脱卤摘要:共轭微孔聚合物(CMPs)由于其结构可设计性和功能多功能性,已成为有前途的有机转化多相光催化剂。然而,受光生激子分离不充分的限制,其光催化效率远低于预期。在此,我们展示了一种氰化策略,通过选择性地将咔唑和氰基分别作为供电子和吸电子单元来促进 CMP 中的电荷载流子分离。由此产生的 CMP 具有 π 扩展的供体 (D)-受体 (A) 共轭结构,赋予它们独特的半导体特性,其中促进了有效的电荷分离和转移以及广泛的可见光吸收。与无氰对应物相比,氰基官能化的 CMP 显示出优异的光催化效率,例如氯化物的光催化还原脱卤。更突出的是,所设计的 CMP 的完全可回收性以及至少 10 次运行的催化活性而不会损失在氯化物的光催化还原脱卤中的催化性能,这证明了它们的稳健性和可持续性。DOI:10.1039/d1cy01386f
-
作为产物:描述:氯苯 在 2,2'-联吡啶 、 aluminum (III) chloride 、 氯化镍二甲氧基乙烷 、 lithium chloride 作用下, 以 二氯甲烷 、 N,N-二甲基乙酰胺 为溶剂, 25.0~40.0 ℃ 、500.01 kPa 条件下, 反应 0.07h, 生成 2'-氯代联苯乙酮参考文献:名称:微电流反应器(μ-EFR)系统,用于超快速的芳烃合成和达卡他韦的制造†摘要:世界卫生组织(世卫组织)已将对称的芳烃daclatasvir(DCV)列为人类健康的基本药物之一。DCV制造通常在多个位置以非连续或“批量”方式进行,并且由于生产时间长(3-10天)而受到严重限制,从而导致无法负担(非常昂贵)并中断潜在的供应链。在这里,我们报告了整个过程系统,包括开发了一种新型电流反应器,该反应器在铜电极上包含带图案的电沉积Ni或Pt纳米粒子,用于无助氧化剂/无氧化剂的超快过程中的C-C偶联反应。用于对称的取代/未取代的联苯合成。该方法进一步扩展到了新一代商业批量合成路线,可在33.2分钟内连续流动超快达克他韦合成。我们设想,这种微电子流反应器(μ-EFR)系统平台将大大促进连续μ流精细化工制造,多步反应序列,反应设计设备和实时提取的发展。DOI:10.1039/c9cc06127d
文献信息
-
Novel anthelmintic and insecticidal compositions申请人:——公开号:US20040171650A1公开(公告)日:2004-09-02The present invention relates to novel anthelmintic and insecticidal compositions in general, and, more specifically, compositions containing triazole derivatives as active ingredients.本发明涉及一般的新的驱虫和杀虫组合物,更具体地说,是含有三唑衍生物作为活性成分的组合物。
-
Synthesis of xylal‐ and arabinal‐based crown ethers and their application as asymmetric phase transfer catalysts作者:Tamás Nemcsok、Zsolt Rapi、Péter Bagi、György Keglevich、Péter BakóDOI:10.1002/chir.23149日期:2020.1from l‐ and d‐xylose, and l‐ and d‐arabinose, respectively. These monosaccharide‐based chiral macrocycles were tested as phase transfer catalysts in a few asymmetric reactions. The xylal‐based crown compounds proved to be efficient catalysts in a few liquid‐liquid phase reactions. The epoxidation of trans‐chalcone and the Darzens condensation of α‐chloroacetophenone with benzaldehyde took place with基于arabinal新xylal-和单氮杂-15-冠醚5合成从开始升-和ð木糖,和升和- d分别-arabinose。这些基于单糖的手性大环化合物在一些不对称反应中作为相转移催化剂进行了测试。在一些液相-液相反应中,基于木糖的冠状化合物被证明是有效的催化剂。反式的环氧化α-氯苯乙酮与苯甲醛的查尔酮和达岑(Darzens)缩合反应具有完全非对映选择性,分别高达ee的77%和ee的58%。发现查尔酮和α-氯乙酰苯的芳香环中的取代基对对映选择性有影响。最高的ee值来自4-氯查尔酮的环氧化(81%ee)和2-萘基类似物的反应(96%ee),而4-苯基-α-氯乙酰苯与苯甲醛的Darzens缩合反应,检测到的最大ee为91%。冠环中单糖单元的构型影响合成的环氧酮的绝对构型。
-
Structural improvement of new thiazolyl-isatin derivatives produces potent and selective trypanocidal and leishmanicidal compounds作者:Luiz Alberto Barros Freitas、Aline Caroline da Silva Santos、Gedália de Cássia Silva、Franciely Nayara do Nascimento Albuquerque、Elis Dionísio Silva、Carlos Alberto de Simone、Valéria Rêgo Alves Pereira、Luiz Carlos Alves、Fabio André Brayner、Ana Cristina Lima Leite、Paulo André Teixeira de Moraes GomesDOI:10.1016/j.cbi.2021.109561日期:2021.8Thus, compounds 2l and 2m showed dual in vitro trypanosomicidal and leishmanicidal activities. A structural activity relationship study showed that thiazolyl-isatin derivatives from phenyl-thiosemicarbazone (2a-m) were, in general, more active than thiazolyl-isatin derivatives from thiosemicarbazone (1a-g). Crystallography studies revealed a different configuration between series 1a-g and 2a-m. The configuration被忽视的疾病是一组主要发生在热带气候国家的传染性疾病。在这一群体中,恰加斯病和利什曼病最为突出,被认为是对全球健康的威胁。这些疾病的治疗是有限的。因此,需要针对这些疾病的新疗法。从这个意义上说,我们的提议包括开发两个系列的化合物,使用杂环靛红和噻唑的分子杂交。靛红和噻唑环是多种生物疾病(包括抗寄生虫疾病)的重要支架。在本文中,噻唑基-靛红是由各自的缩氨基硫脲或苯基-缩氨基硫脲合成的,这些新的噻唑基-靛红对锥鞭毛体有毒,而不影响巨噬细胞的活力。从这个系列,化合物2e(IC 50 = 4.43 μM)、2j (IC 50 = 2.05 μM)、2l (IC 50 = 4.12 μM) 和2m (1.72 μM) 对锥鞭毛体形式表现出最佳的抗克氏锥虫活性,其选择性指数高于苯并硝唑(BZN)。化合物2j、2l和2m能够诱导与锥体鞭毛体坏死相容的显着标记。扫描电子显微镜分析表明,用来自IC 50的化合物2m处理的T
-
Electrochemical preparation of α,α′-dicarbonylselenides作者:Marı́a Dolores Otero、Belen Batanero、Fructuoso BarbaDOI:10.1016/j.tet.2004.03.068日期:2004.5A facile preparation of α,α′-dicarbonylselenides has been performed by reaction of α-carbonyl selenocyanates with an enolate, electrogenerated by reduction of a carbon–halogen bond.
-
Selective Asymmetric Transfer Hydrogenation of α-Substituted Acetophenones with Bifunctional Oxo-Tethered Ruthenium(II) Catalysts作者:Yamato Yuki、Taichiro Touge、Hideki Nara、Kazuhiko Matsumura、Mitsuhiko Fujiwhara、Yoshihito Kayaki、Takao IkariyaDOI:10.1002/adsc.201701227日期:2018.2.1A practical method for the asymmetric transfer hydrogenation of α‐substituted ketones was developed utilizing oxo‐tethered N‐sulfonyldiamine‐ruthenium complexes. Reduction by HCO2H and HCO2K in a mixed solvent of EtOAc/H2O allowed for the selective synthesis of halohydrins from 2‐bromoacetophenone (98%) and 2‐chloroacetophenone (>99%), leading to suppressed undesired side reactions stemming from formylation
表征谱图
-
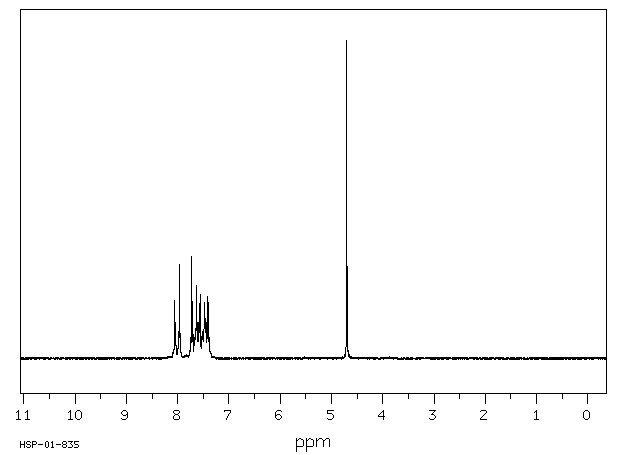
氢谱1HNMR
-
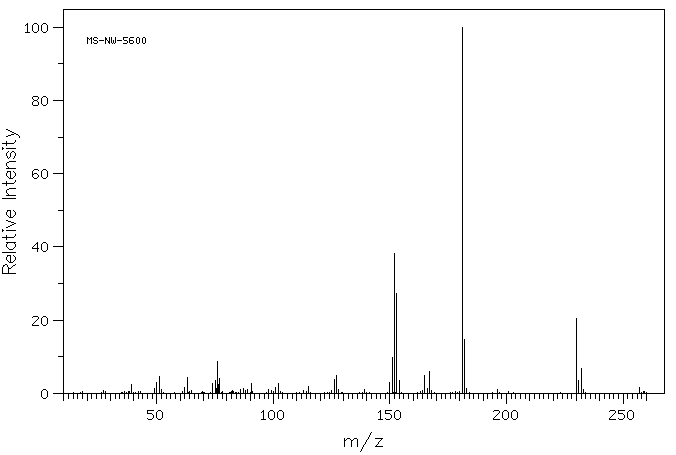
质谱MS
-
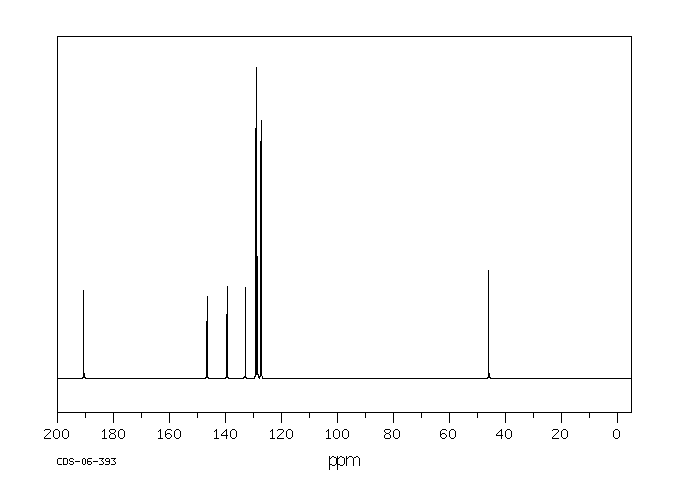
碳谱13CNMR
-
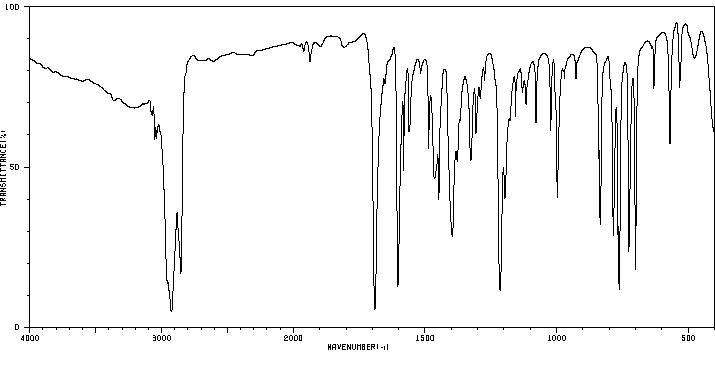
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷