2-苄基苯甲醇 | 1586-00-1
中文名称
2-苄基苯甲醇
中文别名
2-苄基苄醇
英文名称
2-(benzyl)benzyl alcohol
英文别名
2-Benzylbenzyl alcohol;(2-benzylphenyl)methanol
CAS
1586-00-1
化学式
C14H14O
mdl
MFCD00004627
分子量
198.265
InChiKey
GTKWHQWJTVWORA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37-41 °C
-
沸点:154 °C (1 mmHg)
-
密度:1.0074 (rough estimate)
-
稳定性/保质期:
与强氧化剂反应。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:15
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2906299090
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | O-Benzylbenzyl Alcohol 97% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 1586-00-1 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1586-00-1 | O-benzylbenzyl Alcohol | 97 | 216-442-4 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Avoid runoff into storm sewers and ditches which lead to waterways.
Clean up spills immediately, observing precautions in the Protective Equipment section. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1586-00-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: alcohol-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 154 deg C @ 1.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H14O
Molecular Weight: 198.26
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1586-00-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
O-benzylbenzyl Alcohol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1586-00-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1586-00-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1586-00-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-苄基苯甲酸 2-Benzylbenzoic acid 612-35-1 C14H12O2 212.248 1-苯基-1,3-二氢-2-苯并呋喃 1-phenyl-1,3-dihydroisobenzofuran 7111-66-2 C14H12O 196.249 2-苄基苯甲酸甲酯 methyl 2-benzylbenzoate 6962-60-3 C15H14O2 226.275 2-(苄基)苯甲醛 2-benzylbenzaldehyde 32832-95-4 C14H12O 196.249 邻苯甲酰苯甲酸 2-Benzoylbenzoic acid 85-52-9 C14H10O3 226.232 2-苄基苯甲酸乙酯 ethyl 2-benzylbenzoate 1585-99-5 C16H16O2 240.302 2-甲基二苯甲酮 2-Methylbenzophenone 131-58-8 C14H12O 196.249 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苄基-2-(甲氧基甲基)苯 1-(methoxymethyl)-2-(phenylmethyl)benzene 108683-22-3 C15H16O 212.291 —— Bis-<2-benzyl-benzyl>-aether 96217-05-9 C28H26O 378.514 苄基甲苯 2-benzyltoluene 713-36-0 C14H14 182.265 2-苄基苯甲酸 2-Benzylbenzoic acid 612-35-1 C14H12O2 212.248 9,10-二氢蒽 9,10-dihydroanthracene 613-31-0 C14H12 180.249 —— cyclotribenzylene 38059-10-8 C21H18 270.374 —— 2-Benzylbenzyl methanesulfonate 1026077-03-1 C15H16O3S 276.356 2-(苄基)苯甲醛 2-benzylbenzaldehyde 32832-95-4 C14H12O 196.249 2-苄基苄基溴 2-benzylbenzyl bromide 55276-42-1 C14H13Br 261.161 1-苄基-2-(氯甲基)苯 o-benzylbenzyl chloride 7510-28-3 C14H13Cl 216.71 (2-苄基苯基)-乙腈 2-(2-benzylphenyl)acetonitrile 1586-03-4 C15H13N 207.275 - 1
- 2
反应信息
-
作为反应物:描述:2-苄基苯甲醇 在 aluminum oxide 、 sodium hydroxide 、 pyridinium chlorochromate 作用下, 以 1,4-二氧六环 、 乙醇 、 正戊烷 为溶剂, 反应 4.33h, 生成 苄基甲苯参考文献:名称:Guth, Michael; Kirmse, Wolfgang, Acta Chemica Scandinavica, 1992, vol. 46, # 7, p. 606 - 613摘要:DOI:
-
作为产物:参考文献:名称:Chemistry and Stereochemistry of Benzyl-Benzyl Interactions in MH+ Ions of Dibenzyl Esters upon Chemical Ionization and Collision-induced Dissociation Conditions摘要:Isobutane chemical ionization mass spectra of dibenzyl esters of a wide variety of aliphatic, olefinic, alicyclic and aromatic dicarboxylic acids exhibit abundant m/z 181 C14H13+ ions, indicating a highly general rearrangement process involving the formation of a new bond between the two benzyl groups. An extensive collision-induced dissociation and deuterium labeling study suggested that these ions are an almost equimolar mixture of isomeric alpha-o-tolylbenzyl, alpha-p-tolylbenzyl and p-benzylbenzyl cation structures, and this composition is identical for all the diesters examined This structural assignment of the C14H13+ ions suggests a mechanistic pathway for their generation, based on the formation of the new bond between tbe benzyl methylene group of the protonated benzoxycarbonyl and the phenyl ring of the otter ester moiety via pi- (and/or ion-neutral) and a-complexes. Stereoisomeric diesters show an unusual steric effect: trans-isomers give rise to much more abundant C14H13+ ions than the cis counterparts, This behavior is explained by stabilized proton-bridged structures of the MH+ ions of the cis-isomers. (C) 1997 by John Wiley & Sons, Ltd.DOI:10.1002/(sici)1096-9888(199705)32:5<515::aid-jms504>3.0.co;2-b
-
作为试剂:参考文献:名称:Benzylidene rhodanines摘要:本发明提供了一种新型的苄亚胺硫代缩苹果酸,它们作为治疗或预防与β-淀粉样肽相关疾病的药物很有用。本发明还提供了治疗或预防阿尔茨海默病的方法,该方法包括向需要的哺乳动物施用有效量的本发明的一种或多种苄亚胺硫代缩苹果酸。公开号:US05747517A1
文献信息
-
[EN] N-PYRAZOLYL CARBOXAMIDES AS CRAC CHANNEL INHIBITORS<br/>[FR] CARBOXAMIDES N-PYRAZOLYL EN TANT QU'INHIBITEURS DU CANAL CRAC申请人:GLAXO GROUP LTD公开号:WO2010122089A1公开(公告)日:2010-10-28The present invention relates to amide compounds, processes for their preparation, pharmaceutical compositions containing these compounds and to their use in the treatment of disorders, conditions or disorders such as allergic disorders, inflammatory disorders and disorders of the immune system.本发明涉及酰胺化合物,其制备方法,含有这些化合物的药物组合物,以及它们在治疗过敏性疾病、炎症性疾病和免疫系统疾病等疾病、状况或障碍中的应用。
-
A novel synthesis of (di)-benzazocinones via an endocyclic N-acyliminium ion cyclisation作者:Frank D. King、Abil E. Aliev、Stephen Caddick、D. A. TocherDOI:10.1039/c0ob00559b日期:——The triflic acid-mediated endocyclic N-acyliminium ion cyclisation provides a facile synthesis of (di)-benzazocinones. On reduction of the 10-phenyl derivative, an unusually non-polar tertiary alkylamine was obtained.
-
Substituted tricyclics
-
[EN] TRIAZOLOPYRIDINE INHIBITORS OF MYELOPEROXIDASE<br/>[FR] INHIBITEURS, À BASE DE TRIAZOLOPYRIDINE, DE LA MYÉLOPEROXYDASE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2017040449A1公开(公告)日:2017-03-09The present invention provides compounds of Formula (I): wherein A is as defined in the specification, and compositions comprising any of such novel compounds. These compounds are myeloperoxidase (MPO) inhibitors and/or eosinophil peroxidase (EPX) inhibitors, which may be used as medicaments.
-
Silicon-Based Cross-Coupling Reagent and Production Method of Organic Compound Using the Same申请人:Nakao Yoshiaki公开号:US20090069577A1公开(公告)日:2009-03-12In one embodiment of the present invention, a silicon-based cross-coupling reagent is disclosed which is a highly stable tetraorganosilicon compound allowing for a cross-coupling reaction under mild reaction conditions without using fluoride ions, transition metal promoter, or strong bases, and the residue of the silicon reagent can be recovered and reused. The silicon-based cross-coupling reagent is a silicon compound in which an o-hydroxymethylphenyl group is connected to a silicon atom for intramolecular activation.
表征谱图
-
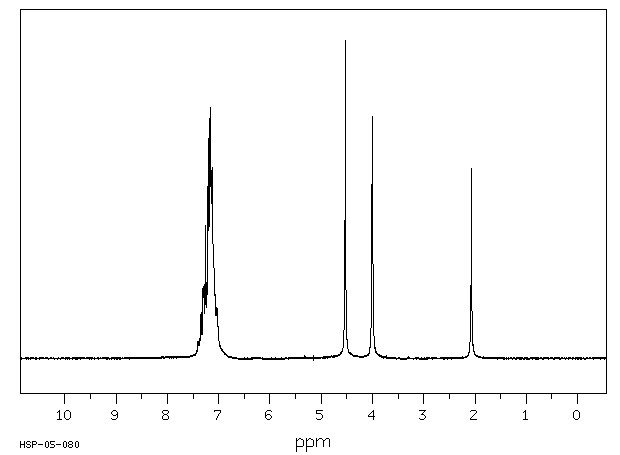
氢谱1HNMR
-
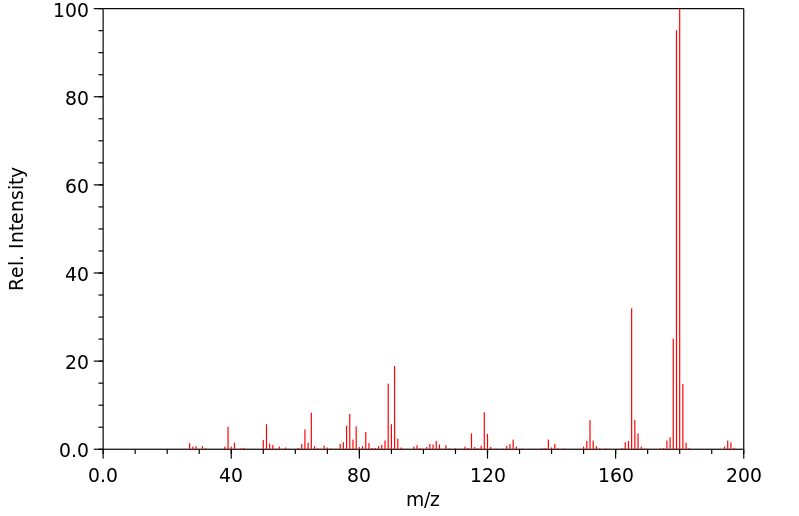
质谱MS
-
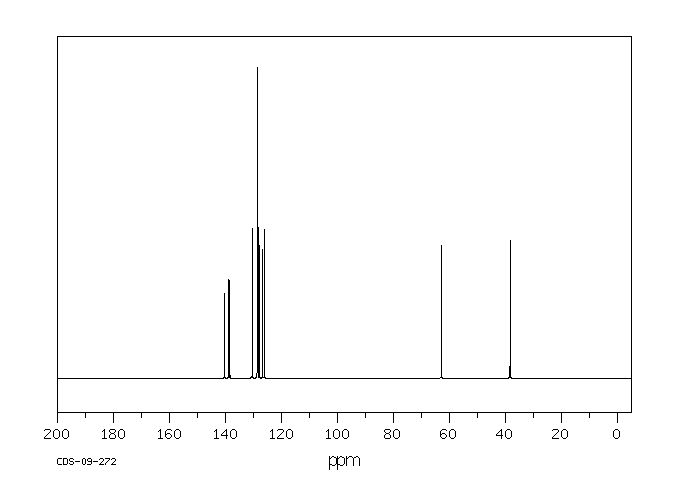
碳谱13CNMR
-
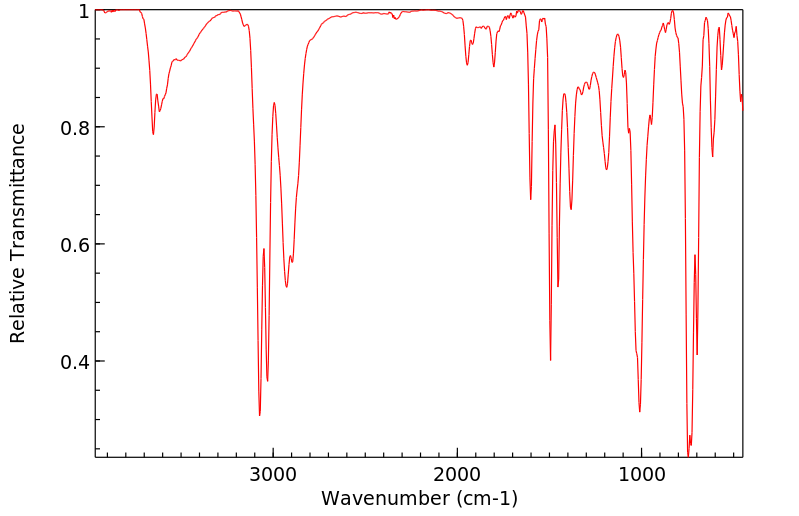
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫