对溴丁氧基苯 | 39969-57-8
中文名称
对溴丁氧基苯
中文别名
1-溴-4-丁氧基苯
英文名称
1-bromo-4-butoxybenzene
英文别名
1-bromo(4-butyloxy)benzene
CAS
39969-57-8
化学式
C10H13BrO
mdl
——
分子量
229.117
InChiKey
BOUVKHWPQNEXTO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79-80 °C
-
沸点:138-142 °C(Press: 8-10 Torr)
-
密度:1.3563 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2909309090
-
安全说明:S24/25
-
储存条件:存储条件:2-8℃,干燥且密封。
SDS
| Name: | 1-Bromo-4-butoxybenzene 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 39969-57-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 39969-57-8 | 1-Bromo-4-butoxybenzene | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 39969-57-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless - pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H13BrO
Molecular Weight: 229
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide, bromine.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 39969-57-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Bromo-4-butoxybenzene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 39969-57-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 39969-57-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 39969-57-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁氧基苯 4-phenoxybutane 1126-79-0 C10H14O 150.221
反应信息
-
作为反应物:描述:对溴丁氧基苯 在 bis-triphenylphosphine-palladium(II) chloride 仲丁基锂 、 potassium carbonate 作用下, 以 四氢呋喃 、 正己烷 、 环己烷 、 Solmix A-11 、 水 、 甲苯 为溶剂, 反应 12.0h, 生成 2,3-difluoro-4-(4-butoxyphenyl)phenylboronic acid参考文献:名称:TETRACYCLIC LIQUID CRYSTALLINE COMPOUND HAVING LATERAL FLUORINE, LIQUID CRYSTAL COMPOSITION AND LIQUID CRYSTAL DISPLAY ELEMENT摘要:公开号:EP2305627B1
-
作为产物:描述:苯 在 sodium chlorite 、 Co(dmgBF2)2(CH3CN)2 、 3-cyano-N-methyl-quinolinium perchlorate 、 manganese(III) acetylacetonate 、 sodium bromide 作用下, 以 二氯甲烷 、 乙腈 为溶剂, 反应 6.0h, 生成 对溴丁氧基苯参考文献:名称:通过光催化氢-演化交叉偶联反应进行苯CHH醚化摘要:可以通过光催化作用和钴催化作用的协同结合,通过苯的C–H键与醇的O–H键之间的直接偶联以及氢的释放来构建芳基醚。利用由3-氰基-1-甲基喹啉光催化剂和钴肟组成的双催化剂体系,完成了芳烃与各种醇的分子间醚化作用以及3-苯基丙醇的分子内烷氧基化反应并形成了色烷。这些反应在非常温和的条件下进行,唯一的副产物是当量的氢气。DOI:10.1021/acs.orglett.7b00463
文献信息
-
PROCESS FOR THE REMOVAL AND RETURN OF A CATALYST TO A LIQUID PHASE MEDIUM申请人:PHOSPHONICS LTD公开号:US20150299229A1公开(公告)日:2015-10-22A process for the selective removal of a component from a liquid phase and subsequently returning the component to a liquid phase is disclosed. A novel compound of formula (I) [SUP]-[[L]-[G]]a (I) in which L is a linking group, G is an aryl group having a leaving group LG selected from Cl, Br, I, sulfonate such as triflate, a diazo group, a nitrile, an ester and an alkoxy group and substituent Q is selected from H, NR2, OR, C02R, F, Cl, N02 CN and SUP is a support having a plurality of groups -[L]-[G] bound to the support is contacted with the liquid phase to bind the component to the compound I thereby forming a captured component which is separated from and may be returned to the liquid phase. The compound I is especially useful in binding homogeneous catalysts to remove it from a reaction medium and selectively returning the catalyst to the reaction medium at a later stage. The compound is particularly useful for cross-coupling reactions, for example in Suzuki reactions.揭示了一种从液相中选择性去除组分并随后将该组分返回到液相的方法。公开了一种具有式(I)的新化合物[SUP]-[[L]-[G]]a (I),其中L是连接基团,G是一种芳基团,具有从Cl、Br、I、磺酸盐(如三氟甲磺酸盐)、重氮基团、腈基、酯基和烷氧基中选择的离去基团LG,取代基Q从H、NR2、OR、C02R、F、Cl、N02、CN中选择,SUP是具有多个与支持物相结合的基团-[L]-[G]的支持物,与液相接触以将组分结合到化合物I中,从而形成被捕获的组分,该组分与液相分离并可以返回到液相中。化合物I在将均相催化剂结合以将其从反应介质中去除,并在后续阶段选择性地将催化剂返回到反应介质中方面特别有用。该化合物特别适用于交叉偶联反应,例如在铃木反应中。
-
<i>Ex Situ</i> Generation of Stoichiometric and Substoichiometric <sup>12</sup>CO and <sup>13</sup>CO and Its Efficient Incorporation in Palladium Catalyzed Aminocarbonylations作者:Philippe Hermange、Anders T. Lindhardt、Rolf H. Taaning、Klaus Bjerglund、Daniel Lupp、Troels SkrydstrupDOI:10.1021/ja200818w日期:2011.4.20CO-precursor led to the development of a new solid, stable, and easy to handle source of CO for chemical transformations. The synthesis of this CO-precursor also provided an entry point for the late installment of an isotopically carbon-labeled acid chloride for the subsequent release of gaseous [(13)C]CO. In combination with studies aimed toward application of CO as the limiting reagent, this method provided使用简单的密封两室系统实现了异位生成一氧化碳 (CO) 及其在钯催化的羰基化反应中的有效结合的新技术。CO 的异位生成是通过钯催化的叔酰氯脱羰使用源自 Pd(dba)(2) 和 P(tBu)(3) 的催化剂产生的。使用新戊酰氯作为 CO 前体的初步研究为仅使用 1.5 当量的 CO 对 2-吡啶基甲苯磺酸酯衍生物进行氨基羰基化提供了另一种方法。 酰氯 CO 前体的进一步设计导致开发了一种新的固体、稳定、并且易于处理用于化学转化的 CO 源。这种 CO 前体的合成也为后期安装同位素碳标记的酰氯以随后释放气态 [(13)C]CO 提供了切入点。结合旨在应用 CO 作为限制剂的研究,该方法提供了高效的钯催化氨基羰基化,CO 结合率高达 96%。异位生成的 CO 和双室系统在几种药物化合物的合成中进行了测试,所有这些化合物都被标记为 [(13)C] 羰基对应物,基于限制 CO 的产率从良好到极好。
-
Access to Aryl and Heteroaryl Trifluoromethyl Ketones from Aryl Bromides and Fluorosulfates with Stoichiometric CO作者:Martin B. Johansen、Oliver R. Gedde、Thea S. Mayer、Troels SkrydstrupDOI:10.1021/acs.orglett.0c01117日期:2020.6.5trifluoromethyl ketones starting from readily accessible aryl bromides and fluorosulfates, the latter easily prepared from the corresponding phenols. The methodology utilizes low pressure carbon monoxide generated ex situ from COgen to generate Weinreb amides as reactive intermediates that undergo monotrifluoromethylation affording the corresponding aromatic trifluoromethyl ketones (TFMKs) in good
-
[EN] CERAMIDE GALACTOSYLTRANSFERASE INHIBITORS FOR THE TREATMENT OF DISEASE<br/>[FR] INHIBITEURS DE LA CÉRAMIDE GALACTOSYLTRANSFÉRASE POUR LE TRAITEMENT DE MALADIES申请人:BIOMARIN PHARM INC公开号:WO2017214505A1公开(公告)日:2017-12-14Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments containing such compounds, and methods of using such compounds to treat or prevent diseases or disorders associated with the enzyme ceramide galactosyltransferase (CGT), such as, for example, lysosomal storage diseases. Examples of lysosomal storage diseases include, for example, Krabbe disease and Metachromatic Leukodystrophy.本文描述了化合物、制备这种化合物的方法、含有这种化合物的药物组合物和药物、以及使用这种化合物治疗或预防与酶神经鞘糖脂转移酶(CGT)相关的疾病或紊乱的方法,例如溶酶体贮积症。溶酶体贮积症的例子包括 Krabbe 病和白质变性白血病。
-
Synthesis of Benzyl <i>C</i> -Analogues of Dapagliflozin as Potential SGLT2 Inhibitors作者:Ramesh Mukkamala、Roshan Kumar、Sanjay K. Banerjee、Indrapal Singh AidhenDOI:10.1002/ejoc.202000025日期:2020.3.31A convenient synthetic strategy has been developed for the synthesis of C‐benzyl analogues of dapagliflozin. The In vitro sodium‐glucose co‐transporters (SGLT1 and SGLT2) inhibition activity of all new compounds exhibits promising results, particularly, compound 14 emerged as the most potent SGLT2 inhibitor with the best selectivity for inhibition of SGLT2 (IC50:0.64 nm) over SGLT1 (IC50: 500 nm) as
表征谱图
-
氢谱1HNMR
-
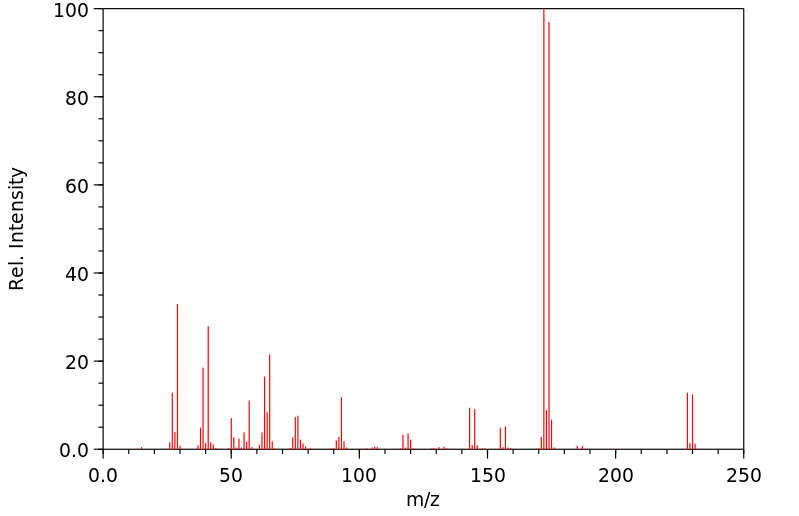
质谱MS
-
碳谱13CNMR
-
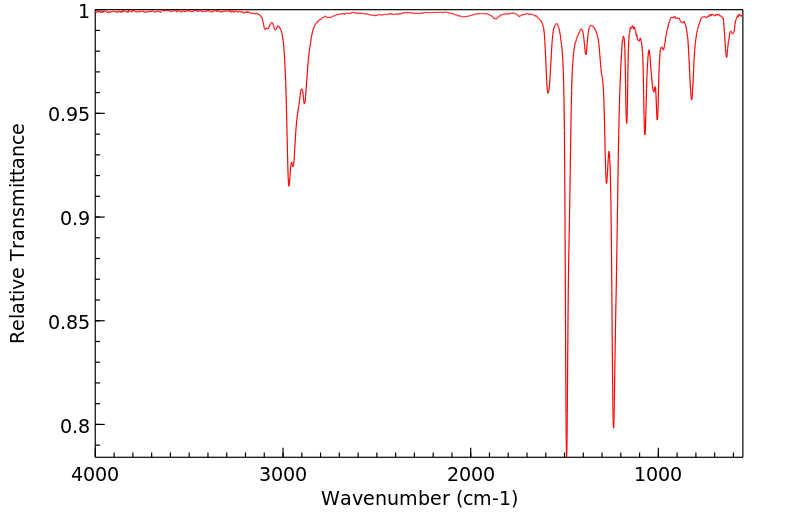
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯