sodium sulfanilate | 515-74-2
中文名称
——
中文别名
——
英文名称
sodium sulfanilate
英文别名
sodium p-aminobenzenesulfonate;sodium 4-aminobenzenesulfonate;sulfanilic acid sodium salt;1-aminobenzene-4-sulphonic acid sodium salt;Sodium;4-sulfonatoaniline;sodium;4-sulfonatoaniline
CAS
515-74-2
化学式
C6H6NO3S*Na
mdl
——
分子量
195.174
InChiKey
KSVSZLXDULFGDQ-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:DryPowder, Liquid; Liquid
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-2.82
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:91.6
-
氢给体数:1
-
氢受体数:4
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
储存条件:1. 储存在阴凉、干燥且通风良好的仓库中。远离火源和热源,避免阳光直射,并确保包装密封。应与酸类及食用化学品分开存放,禁止混储。存储区域需准备合适的材料以处理泄漏。 2. 使用铁桶或麻袋进行内衬塑料袋包装,每桶(袋)重25公斤或50公斤。存放在阴凉、干燥且通风的地方,防止受潮导致变质。搬运时要轻拿轻放,避免碰撞。
SDS
制备方法与用途
反应信息
-
作为反应物:描述:sodium sulfanilate 在 盐酸 、 ammonium hydroxide 、 氯化亚砜 、 水 、 N,N-二甲基甲酰胺 、 sodium hydroxide 作用下, 以 四氢呋喃 、 乙醇 、 水 为溶剂, 反应 5.0h, 生成 磺胺参考文献:名称:超越碱性:在细胞培养中发现具有强活性的非碱性DENV-2蛋白酶抑制剂摘要:病毒丝氨酸蛋白酶NS2B-NS3是针对登革热病毒和其他黄病毒的药物发现的有希望的靶标之一。蛋白酶的分子识别偏爱偏向于碱性,带正电荷的部分作为底物和抑制剂,这会导致药代动力学方面的问题以及与宿主蛋白酶(如凝血酶)的脱靶相互作用。我们在这里介绍了专门针对黄病毒病毒的不带电荷的小分子抑制剂的发现和开发的努力结果。发现这些化合物的关键因素是登革热蛋白酶DENV2proHeLa系统的细胞报告基因检测。广泛的结构-活性关系探索在病毒复制测定中产生了具有亚微摩尔活性的新型苯甲酰胺衍生物(EC 500.24μM),针对脱靶蛋白酶的选择性和可忽略的细胞毒性。与大多数以前公开的针对活性部位的黄病毒蛋白酶抑制剂相比,该结构类别的药物相似性增强,并且包括有望用于进一步的临床前开发的候选药物。DOI:10.1021/acs.jmedchem.0c02042
-
作为产物:描述:参考文献:名称:用于选择性检测纯水溶液中 Al3+ 的荧光化学传感器的功能导向合成:纸条检测和 DNA 生物成像摘要:基于荧光的传感器在用于选择性检测不同分析物的快速响应设备中已获得普及。它们中的大多数是有机共轭支架,通常在水中缺乏溶解性,从而限制了它们的应用。在此,我们报告了一种完全水溶性的水杨醛磺化衍生物,设计用于选择性检测纯水溶液中的铝离子。研究了S1和 π 共轭磺基苯胺酸类似物SA1的光物理性质,并筛选了它们的选择性、灵敏度、结合模式和在点纸测试以及生物成像中的应用。两种传感器分子都表现出对 Al 3+ 的高度选择性,而不是其他金属离子。他们显示了大 Kb值接近 10 4,化学计量比为 3:1,由 BH 和定量图证明,随后通过 ESI-MS 验证。随着 Al 3+浓度的增加,观察到异常的荧光响应,S1和SA1 的检测限为 0.827 μM 和 1.67 μM分别。根据世界卫生组织的指导方针,这些值远低于饮用水中铝 (III) 的允许限值。通过使用 B3LYP 混合功能对传感器及其复合物的优化几何形状进行DOI:10.1016/j.jphotochem.2021.113699
-
作为试剂:参考文献:名称:Chinon-Amin-Reaktionen, 8. Mitt.1) Synthese von 6-Amino-benzo[f]chinoxalin摘要:DOI:10.1002/ardp.19833161016
文献信息
-
Water-Soluble and Recyclable Cyclopalladated Ferrocenylimine for Suzuki Coupling Reaction作者:Yang Li、Zhihua Fu、Lu Rao、Li Wang、Rui Li、Tiesheng Li、Yangjie WuDOI:10.1002/jccs.201300353日期:2014.3A series of new water‐soluble cyclopalladated ferrocenylimines were designed and prepared. They were efficient catalyst for Suzuki coupling reactions of aryl bromides and phenylboronic acid in neat water under ambient atmosphere. Among of these catalysts, the catalyst (C2D) could be reused for 6 times for the Suzuki coupling reaction of 4‐bromotoluene with phenylboronic acid in EtOH/H2O under ambient
-
光応答性化合物及び該化合物を有効成分とする光応答性分散剤申请人:国立研究開発法人産業技術総合研究所公开号:JP2019077679A公开(公告)日:2019-05-23【課題】分散剤を含む分散液に光を短時間照射することで、分散質が分散液中で分散と凝集を繰り返すことができる分散剤の提供。【解決手段】一般式(I)で表される光応答性化合物を有効成分とする分散剤。(X、Yは、(1)X:C=O、Y:NH(2)X:O、Y:C=O(3)X:C=O、Y:Oで示される組み合わせから選択される、アミド結合又はエステル結合;Aは、スルホン酸、スルホン酸塩、カルボン酸又はカルボン酸塩からなるアニオン性の親水性官能基、もしくはヒドロキシル基又はポリエチレングリコール基からなるノニオン性の親水性官能基、もしくはアルキル基又はフルオロアルキル鎖からなる非親水性官能基;nは1以上3以下の整数を表す。)【選択図】なし
-
Fullerene-Catalyzed Reduction of Azo Derivatives in Water under UV Irradiation作者:Yong Guo、Wengang Li、Jingjing Yan、Basem Moosa、Ma'an Amad、Charles J. Werth、Niveen M. KhashabDOI:10.1002/asia.201200701日期:2012.12catalyst for the reduction of azo groups in basic aqueous solution under UV irradiation in the presence of NaBH4. Use of NaBH4 by itself is not sufficient to reduce the azo dyes without the assistance of a metal catalyst such as Pd and Ag. Experimental and theoretical results suggest that C60 catalyzes this reaction by using its vacant orbital to accept the electron in the bonding orbital of azo dyes, which
-
Reductive cleavage of azo compounds catalyzed by commercial zinc dust using ammonium formate or formic acid作者:Shankare Gowda、K Abiraj、D.Channe GowdaDOI:10.1016/s0040-4039(01)02370-x日期:2002.2Azo compounds, both symmetric and unsymmetric, are cleaved to amine(s) by using commercial zinc dust and ammonium formate or formic acid in methanol, tetrahydrofuran or dioxane at room temperature. The reductive cleavage occurs without hydrogenolysis or hydrogenation of reducible moieties, such as -OH, -CH3, -OCH3, -COOH, -COCH3, halogen, etc. The cleavage is very fast, clean, cost effective and high-yielding
-
Facile Transfer Hydrogenation of Azo Compounds to Hydrazo Compounds and Anilines by Using Raney Nickel and Hydrazinium Monoformate作者:H. S. Prasad、Shankare Gowda、D. Channe GowdaDOI:10.1081/scc-120027231日期:2004.12.31Abstract Azo compounds are conveniently reduced to either partially reduced hydrazo compounds or completely reduced anilines by employing Raney nickel in presence of hydrazinium monoformate depending upon reaction conditions. The other reducible moieties like ‒COOH and halogens are tolerated. The reduction process is selective, rapid and high yielding.
表征谱图
-
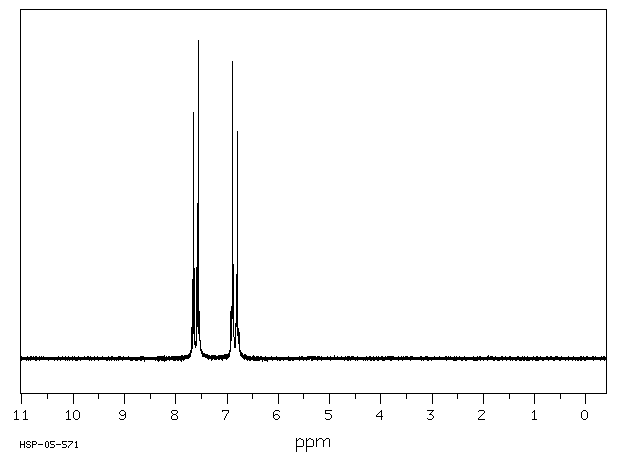
氢谱1HNMR
-
质谱MS
-
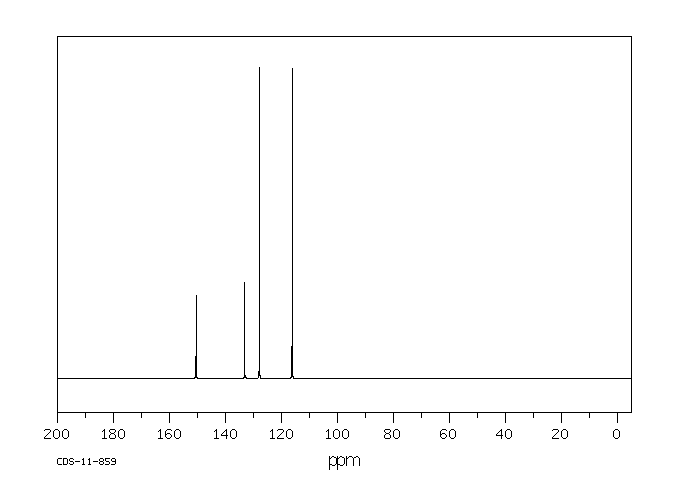
碳谱13CNMR
-
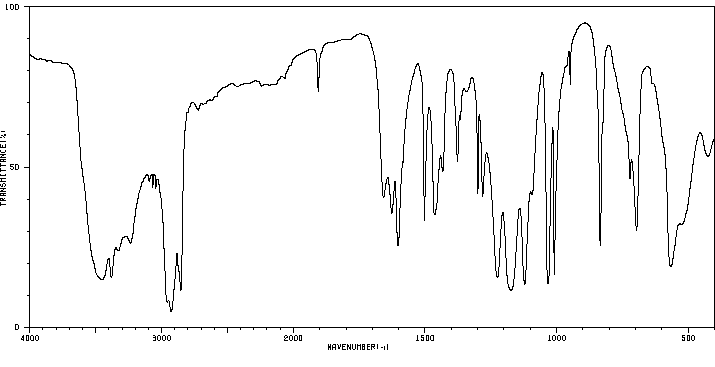
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫