3,4,5-三甲氧基苯甲腈 | 1885-35-4
中文名称
3,4,5-三甲氧基苯甲腈
中文别名
3,4,5-三甲氧基苯腈
英文名称
3,4,5-trimethoxybenzonitrile
英文别名
3,4,5-trimethoxylbenzonitrile
CAS
1885-35-4
化学式
C10H11NO3
mdl
MFCD00001803
分子量
193.202
InChiKey
OSBQUSPVORCDCU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:91-94 °C (lit.)
-
沸点:180-185 °C/10 mmHg (lit.)
-
密度:1.2307 (rough estimate)
-
闪点:180-185°C/10mm
-
溶解度:溶于甲醇
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:51.5
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2926909090
-
RTECS号:DI4965000
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3276
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 3 4 5-Trimethoxybenzonitrile 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1885-35-4 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1885-35-4 | 3,4,5-Trimethoxybenzonitrile | 97 | 217-550-4 |
Risk Phrases: 21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration.
Ingestion:
May cause irritation of the digestive tract. May cause cardiac disturbances. The toxicological properties of this substance have not been fully investigated. May cause central nervous system depression.
Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May cause cardiac abnormalities. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration.
Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
May be partially metabolized to cyanide in the body.
Antidote: Always have a cyanide antidote kit on hand when working with cyanide compounds. Get medical advice to use.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1885-35-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 180 - 185 deg C @ 10.00mmHg
Freezing/Melting Point: 91.00 - 94.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H11NO3
Molecular Weight: 193.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, cyanide fumes, nitrogen oxides (NOx) and ammonia (NH3).
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1885-35-4: DI4965000 LD50/LC50:
Not available.
Carcinogenicity:
3,4,5-Trimethoxybenzonitrile - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 21/22 Harmful in contact with skin and if
swallowed.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
WGK (Water Danger/Protection)
CAS# 1885-35-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1885-35-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1885-35-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
为氮配体
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4,5-三甲氧基甲苯 3,4,5-trimethoxytoluene 6443-69-2 C10H14O3 182.219 3,4,5-三甲氧基苄醇 (3,4,5-trimethoxyphenyl)methanol 3840-31-1 C10H14O4 198.219 —— 3,4,5-trimethoxy styrene 13400-02-7 C11H14O3 194.23 3,4,5-三甲氧基苯甲醛 3,4,5-trimethoxy-benzaldehyde 86-81-7 C10H12O4 196.203 3,4,5-三甲氧基苄胺 3,4,5-trimethoxybenzylamine 18638-99-8 C10H15NO3 197.234 3.4.5-三甲氧基苯乙炔 3,4,5-trimethoxyphenylacetylene 53560-33-1 C11H12O3 192.214 1,2,3-三甲氧基-5-(甲氧基甲基)苯 1,2,3-trimethoxy-5-(methoxymethyl)benzene 75921-68-5 C11H16O4 212.246 (Z)-3,4,5,4',-四甲氧基-3'-羟基二苯乙烯 combrestatin A4 117048-59-6 C18H20O5 316.354 3,4,5-三甲氧基苯甲醛肟 3,4,5-trimethoxybenzaldoxime 39201-89-3 C10H13NO4 211.218 (E)-3,4,5-三甲氧基苯甲醛肟 (E)-3,4,5-trimethoxybenzaldehyde oxime 39201-89-3 C10H13NO4 211.218 —— 5-(azidomethyl)-1,2,3-trimethoxybenzene 133992-56-0 C10H13N3O3 223.232 3,4,5-三甲氧基苯乙酸 3,4,5-trimethoxyphenyl acetic acid 951-82-6 C11H14O5 226.229 —— (E)-1,1-dimethyl-2-(3,4,5-trimethoxybenzylidene)hydrazine 105143-07-5 C12H18N2O3 238.287 —— 3,4,5-Trimethoxybenzaldehyde N,N-dimethylhydrazone 105143-07-5 C12H18N2O3 238.287 3,4,5-三甲氧基苯甲酸 Eudesmic acid 118-41-2 C10H12O5 212.202 3,4,5-三甲氧基苯甲酰氯 3,4,5-Trimethoxybenzoyl chloride 4521-61-3 C10H11ClO4 230.648 3,4,5-三甲氧基苯甲酰胺 3,4,5-trimethoxybenzamide 3086-62-2 C10H13NO4 211.218 —— 3,4,5-Trimethoxybenzaldehyde oxime acetate —— C12H15NO5 253.255 —— 1-phenyl-2-(3,4,5-trimethoxybenzylidene)hydrazine 63452-43-7 C16H18N2O3 286.331 —— N-Phenyl-N'-[1-(3,4,5-trimethoxy-phenyl)-meth-(E)-ylidene]-hydrazine 63452-43-7 C16H18N2O3 286.331 3,4,5-三甲氧基苯甲酸乙酯 ethyl 3,4,5-trimethoxybenzoate 6178-44-5 C12H16O5 240.256 2-(3,4,5-三甲氧基苯基)环氧乙烷 3,4,5-trimethoxystyrene oxide 54767-81-6 C11H14O4 210.23 —— N,N-dimethyl(3,4,5-trimethoxyphenyl)carboxamide 5658-49-1 C12H17NO4 239.271 —— N,3,4,5-tetramethoxybenzamide 25563-19-3 C11H15NO5 241.244 —— 3,4,5-trimethoxybenzoyl azide 42543-45-3 C10H11N3O4 237.215 —— (Z)-N,3,4,5-tetramethoxybenzenecarboximidoyl bromide 140463-00-9 C11H14BrNO4 304.14 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氰基-2,6-二甲氧基苯酚 4-hydroxy-3,5-dimethoxybenzonitrile 72684-95-8 C9H9NO3 179.175 3,4,5-三甲氧基甲苯 3,4,5-trimethoxytoluene 6443-69-2 C10H14O3 182.219 —— 2,3,4,5-Tetramethoxybenzonitrilato 1478593-03-1 C11H13NO4 223.229 3,4,5-三甲氧基苯甲醛 3,4,5-trimethoxy-benzaldehyde 86-81-7 C10H12O4 196.203 3,4,5-三甲氧基苄胺 3,4,5-trimethoxybenzylamine 18638-99-8 C10H15NO3 197.234 3,4,5-三羟基苯甲腈 3,4,5-trihydroxybenzonitrile 38897-26-6 C7H5NO3 151.122 3,4,5-三甲氧基苯甲醛肟 3,4,5-trimethoxybenzaldoxime 39201-89-3 C10H13NO4 211.218 —— N,N-dimethyl-1-(3,4,5-trimethoxyphenyl)methanamine 34274-02-7 C12H19NO3 225.288 —— bis(3,4,5-trimethoxylbenzyl)amine 35146-75-9 C20H27NO6 377.437 —— 3,4,5-trimethoxybenzaldehyde N-[-(3,4,5-trimethoxyphenyl)methylidene]hydrazone 7251-01-6 C20H24N2O6 388.42 3',4',5'-三甲氧基苯乙酮 3,4,5-Trimethoxyacetophenone 1136-86-3 C11H14O4 210.23 —— 4-Amino-3',4',5'-trimethoxydiphenylmethane 24891-49-4 C16H19NO3 273.332 3,4,5-三甲氧基硫代苯甲酰胺 3,4,5-Trimethoxy-thiobenzamid 60987-94-2 C10H13NO3S 227.284 3,4,5-三甲氧基苯甲酰胺 3,4,5-trimethoxybenzamide 3086-62-2 C10H13NO4 211.218 —— 2-amino-3,4,5-trimethoxybenzonitrile 184042-07-7 C10H12N2O3 208.217 —— (E)-N-(3,4,5-trimethoxybenzylidene)methanesulfonamide —— C11H15NO5S 273.31 —— phenyl(3,4,5-trimethoxyphenyl)methanone 55363-58-1 C16H16O4 272.301 1,2,3-三甲氧基苯 1,2,3-trimethoxybenzene 634-36-6 C9H12O3 168.192 —— N-hydroxy-3,4,5-trimethoxybenzimidoyl chloride 105677-86-9 C10H12ClNO4 245.663 —— N-methyl-3,4,5-trimethoxybenzamide 55100-33-9 C11H15NO4 225.244 —— 4-methoxy-N-(3,4,5-trimethoxybenzyl)aniline 134029-85-9 C17H21NO4 303.358 —— N'-hydroxy-3,4,5-trimethoxybenzimidamide 36957-30-9 C10H14N2O4 226.232 —— ethyl 3,4,5-trimethoxybenzene-1-carboximidate 6383-98-8 C12H17NO4 239.271 1-(4-羟基-3,5-二甲氧基苯基)-1-丙酮 1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one 5650-43-1 C11H14O4 210.23 —— (4-hydroxy-3,5-dimethoxyphenyl)(phenyl)methanone 93899-24-2 C15H14O4 258.274 1,2,3,4,5-戊甲氧基苯 1,2,3,4,5-pentamethoxybenzene 13909-75-6 C11H16O5 228.245 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Exploring the reactivity of nickel complexes in hydrodecyanation reactions摘要:In the present study, the nickel-catalyzed hydrodecyanation of organic cyanides with lithium borohydride as a cheap hydride source has been examined in detail. As precatalysts straightforward nickel complexes modified by tridentate O,N,O'-ligands and triphenylphosphane as co-ligand have been applied. Noteworthy, excellent yields and chemoselectivities were feasible for a variety of organic cyanides at low catalyst loadings and low temperature (70 degrees C) within short reaction time (3 h). (C) 2013 Elsevier B.V. All rights reserved.DOI:10.1016/j.jorganchem.2013.07.068
-
作为产物:描述:3,4,5-三甲氧基苄胺 在 C68H64Cl2N6P2Ru2(4+)*2F6P(1-)*2Cl(1-) 、 caesium carbonate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 以96.3%的产率得到3,4,5-三甲氧基苯甲腈参考文献:名称:N-杂环卡宾-氮-膦螯合的双金属钌(II)络合物催化的胺无受体脱氢为腈摘要:我们已经开发出一种清洁,原子经济且环保的方法,通过将新的双N-杂环碳烯-氮-膦配体R(CNP)2(R = 邻-二甲苯基)与钌前体结合,将胺无接受地脱氢为腈。将[RuCl 2(η 6 -C 6 H ^ 6)] 2。在该系统中,胺的电子和空间因素对反应的影响可忽略不计,并且广泛耐受各种官能团。所有研究的胺都可以优良的选择性转化为腈,收率高达99%。该系统空前的催化性能归因于两个被R(CNP)2螯合的钌中心的协同作用,并且根据通过原位NMR和HRMS发现的活性物种,提出了合理的反应机理。DOI:10.1016/j.jcat.2020.09.005
文献信息
-
From aldehydes to nitriles, a general and high yielding transformation using HOF·CH3CN complex作者:Mira Carmeli、Neta Shefer、Shlomo RozenDOI:10.1016/j.tetlet.2006.10.014日期:2006.12N,N-Dimethylhydrazones of aldehydes undergo a rapid oxidative cleavage to form nitriles in very high yields on reaction with HOF·CH3CN under mild conditions. The reaction is chemoselective and proceeds rapidly without racemization. The nitriles were resistant to further oxidation, even when a large excess of the reagent was employed.醛的N,N-二甲基hydr在与HOF·CH 3 CN的温和条件下反应迅速发生氧化裂解,从而以很高的收率形成腈。该反应是化学选择性的,并且在没有外消旋的情况下迅速进行。即使使用大量过量的试剂,腈也能抵抗进一步的氧化。
-
Nickel-Catalyzed Reversible Functional Group Metathesis between Aryl Nitriles and Aryl Thioethers作者:Tristan Delcaillau、Philip Boehm、Bill MorandiDOI:10.1021/jacs.1c00529日期:2021.3.17We describe a new functional group metathesis between aryl nitriles and aryl thioethers. The catalytic system nickel/dcype is essential to achieve this fully reversible transformation in good to excellent yields. Furthermore, the cyanide- and thiol-free reaction shows high functional group tolerance and great efficiency for the late-stage derivatization of commercial molecules. Finally, synthetic applications
-
Atom-Economical and Tandem Conversion of Nitriles to <i>N</i>-Methylated Amides Using Methanol and Water作者:Bhaskar Paul、Milan Maji、Sabuj KunduDOI:10.1021/acscatal.9b03916日期:2019.11.1A cobalt complex catalyzed tandem conversion of nitrile to N-methylated amide is described using a methanol and water mixture. Using this protocol, several nitriles were directly and efficiently converted to the desired N-methylated amides. Kinetic experiments using H2O18 and CD3OD suggested that water and methanol were the source of the oxygen atom and methyl group, respectively, in the final N-methylated
-
Nickel-Catalyzed Cyanation of Aryl Halides and Hydrocyanation of Alkynes via C–CN Bond Cleavage and Cyano Transfer作者:Hui Chen、Shuhao Sun、Yahu A. Liu、Xuebin LiaoDOI:10.1021/acscatal.9b04586日期:2020.1.17methods to prepare aryl nitriles and vinyl nitriles from aryl halides and alkynes, respectively. Using inexpensive and non-toxic 4-cyanopyridine N-oxide as the cyano shuttle, the methods provide an efficient approach to prepare aryl cyanides and vinyl nitriles under mild and operationally simple reaction conditions with a broad range of functional group tolerance. In hydrocyanation of alkynes, the method
-
Correction: Copper-mediated cyanation of indoles and electron-rich arenes using DMF as a single surrogate作者:Lianpeng Zhang、Ping Lu、Yanguang WangDOI:10.1039/c6ob90012g日期:——
Correction for ‘Copper-mediated cyanation of indoles and electron-rich arenes using DMF as a single surrogate’ by Lianpeng Zhang
et al. ,Org. Biomol. Chem. , 2015,13 , 8322–8329.
表征谱图
-
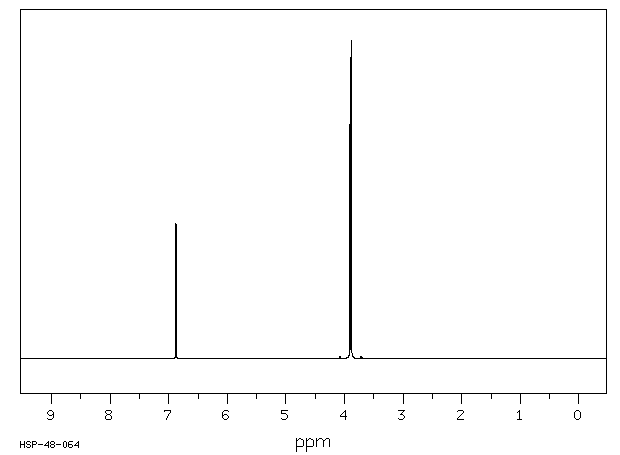
氢谱1HNMR
-
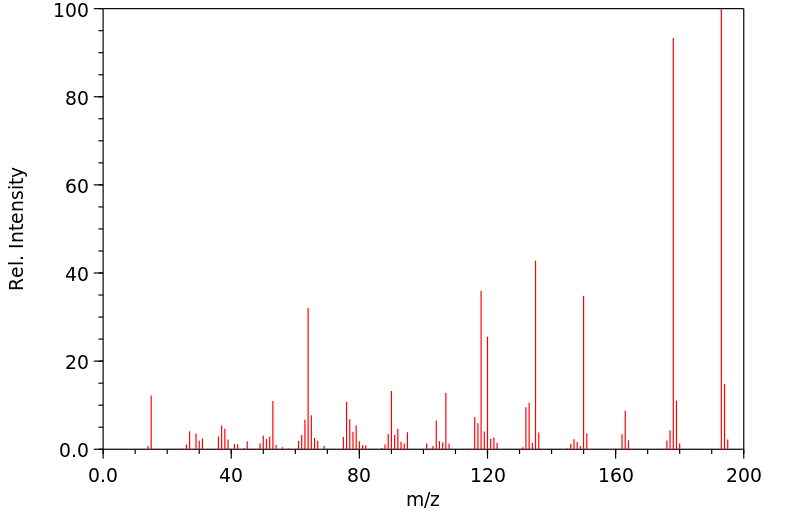
质谱MS
-
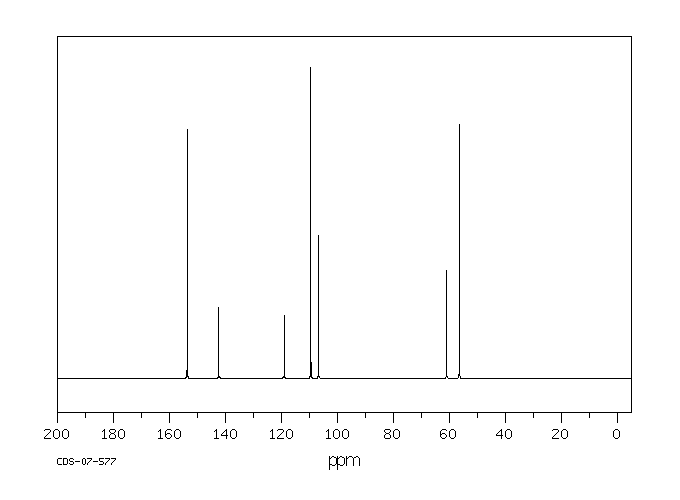
碳谱13CNMR
-
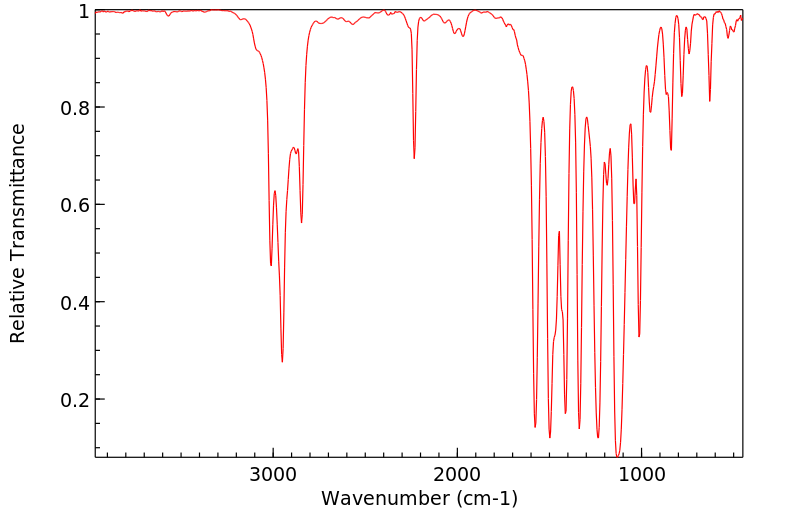
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫