5-甲基-2-呋喃甲醇 | 3857-25-8
中文名称
5-甲基-2-呋喃甲醇
中文别名
5-甲基呋喃-1-甲醇;5-甲糠醇;(5-甲基-2-呋喃基)甲醇
英文名称
2-hydroxymethyl-5-methylfuran
英文别名
5-methylfurfuryl alcohol;5-methyl-2-furanmethanol;(5-methylfuran-2-yl)methanol;(5-methyl-2-furyl)methanol;MFA
CAS
3857-25-8
化学式
C6H8O2
mdl
——
分子量
112.128
InChiKey
VOZFDEJGHQWZHU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:80°C 15mm
-
密度:1.0769
-
溶解度:可溶于氯仿(少许)、乙酸乙酯(少许)、甲醇(少许)
-
LogP:0.66
-
物理描述:Colourless to pale yellow liquid; Sweet caramel-like aroma
-
折光率:1.484-1.490
-
保留指数:958;966;953
-
稳定性/保质期:
避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:33.4
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
危险品运输编号:UN 2810
-
海关编码:2932190090
-
安全说明:S36/37
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:密封储存,应存放在阴凉、干燥的仓库中。
SDS
| Name: | 5-Methyl-2-furanmethanol 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 3857-25-8 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3857-25-8 | 5-Methyl-2-furanmethanol | 97% | unlisted |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
The toxicological properties of this substance have not been fully investigated.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3857-25-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless to yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 80 deg C @ 15 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density: 1.0769
Molecular Formula: C6H8O2
Molecular Weight: 112.0548
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
None reported.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3857-25-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Methyl-2-furanmethanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 3857-25-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3857-25-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3857-25-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
- 烟草: FC, 40。
- 烟草: FC, 40。
(此处为空,暂无内容)
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-呋喃二甲醇 2,5-bis-(hydroxymethyl)furan 1883-75-6 C6H8O3 128.128 糠醇 (2-furyl)methyl alcohol 98-00-0 C5H6O2 98.1014 5-羟甲基糠醛 5-hydroxymethyl-2-furfuraldehyde 67-47-0 C6H6O3 126.112 —— 2-(formyloxy)methyl-5-(hydroxymethyl)furan 1253934-87-0 C7H8O4 156.138 2,5-二甲基呋喃 2,5-dimethylfuran 625-86-5 C6H8O 96.1289 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methoxymethyl-methylfuran 18091-23-1 C7H10O2 126.155 —— 2-(formyloxy)methyl-5-methylfuran 1008130-37-7 C7H8O3 140.139 —— 2-(ethoxymethyl)-5-methylfuran 35901-19-0 C8H12O2 140.182 —— 2-(formyloxy)methyl-5-(hydroxymethyl)furan 1253934-87-0 C7H8O4 156.138 —— 2,5-bis[(formyloxy)methyl]furan 1008130-34-4 C8H8O5 184.149 —— 2-methyl-5-((prop-2-ynyloxy)methyl)furan 99758-19-7 C9H10O2 150.177 5-甲基-2-糠酸 5-methylfuran-2-carboxylic acid 1917-15-3 C6H6O3 126.112 2,5-二甲基呋喃 2,5-dimethylfuran 625-86-5 C6H8O 96.1289 —— 3-(5-Methyl-2-furanylmethoxy)-1-propanol 160858-56-0 C9H14O3 170.208 2-(丁氧基甲基)-5-甲基呋喃 2-butoxymethyl-5-methylfuran 126080-79-3 C10H16O2 168.236 5-羟甲基-2-呋喃甲酸 5-hydroxymethyl-furan-2-carboxylic acid 6338-41-6 C6H6O4 142.111 5-甲醛基呋喃-2-羧酸 5-Formyl-2-furancarboxylic acid 13529-17-4 C6H4O4 140.095 —— 2-(2-Ethoxyethoxy)ethyl 5-methylfurfuryl ether —— C12H20O4 228.288 5-甲基-2-糠基乙酸酯 5-methyl-2-furfuryl acetate 18091-24-2 C8H10O3 154.166 2,5-呋喃二甲酸 furan-2,5-dicarboxylic acid 3238-40-2 C6H4O5 156.095 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Pd催化原位多米诺法直接从碳水化合物中轻量定量生产2,5-二甲基呋喃摘要:已开发出一种原位多米诺法,该方法可高效地将各种己糖直接和温和地转化为生物燃料2,5-二甲基呋喃,几乎可以定量收率,而无需在120°C的正丁醇中分离出不稳定的中间体。聚甲基氢硅氧烷和疏水性Pd / C分别作为氢供体和双官能催化剂。在级联反应中,氘化标记和动力学研究证实了氢化硅烷化过程有利于糖的脱水,并且专门作用于将原位形成的中间体(包括呋喃醇和醛)经DMF脱氧成DMF 。醇类溶剂促进的氢化物转移过程。该催化体系比参与H 2的对应体系更具选择性,并且可以在催化剂负载量仅为0.04 mol%的情况下按比例放大,从而以85%的可比产率得到DMF。此外,Pd(0)被证明是脱氧的活性物质,非均相催化剂具有良好的可回收性,几乎没有元素浸出。DOI:10.1039/c7gc00580f
-
作为产物:描述:5-(溴甲基)呋喃-2-甲醛 在 platinum on carbon 、 氢气 作用下, 以 1,2-二氯乙烷 为溶剂, 50.0 ℃ 、1.1 MPa 条件下, 反应 5.0h, 以53.3%的产率得到5-甲基-2-呋喃甲醇参考文献:名称:钯催化生物质衍生的卤化糠醛的氢化†摘要:非常需要由木质纤维素生物质形成有价值的产物,尤其是候选燃料。据报道,与当前现有的木质纤维素转化方法相比,木质纤维素衍生的卤化糠醛形成非常有效。但是,卤代糠醛是平台化学品,而不是最终用途化学品,当然不是燃料。因此,期望将卤代糠醛转化为燃料,燃料添加剂或其他有价值的化合物的方法。在这项工作中,我们介绍了在碳载钯催化剂上氢化卤代糠醛的方法。与其他催化剂相比,钯催化剂在由卤化糠醛形成5-甲基糠醛(MF)中表现出更好的性能。反应产物使用GC-MS,FT-IR和NMR进行鉴定,并使用GC分析对其进行了定量。用SEM,BET和pH计表征催化剂。考察了催化剂性能和反应参数在MF制备中的作用,以及它们对MF收率和选择性的影响。另外,还检查了催化剂在后续循环中的回收和再利用,以及在卤代糠醛加氢中作为副产物形成的盐酸或氢溴酸的回收。DOI:10.1039/c6ra21472j
文献信息
-
A two-phase system for the clean and high yield synthesis of furylmethane derivatives over –SO<sub>3</sub>H functionalized ionic liquids作者:S. H. Shinde、C. V. RodeDOI:10.1039/c7gc01654a日期:——respective ionic liquids. Among the several preapered ionic liquids, strong acidic imidazolium based butylsulfonic acid (6) showed the best activity with a maximum of 84% yield of condensation product. This strategy offers significantly high yield production of condensation products of furan and furfural as compared to the traditional mineral acid route. The activity and stability of the -SO3H functionalized我们在这里报告了一种新的有效的独特的两相反应系统,用于从糠醛和呋喃中高产率生产三(糠基)甲烷。该策略包括酸性水相(水+ -SO3H官能化的IL)和呋喃相,它们显着抑制了聚合物的形成,从而提高了三(呋喃基)甲烷的收率。呋喃既用作反应物又用作萃取溶剂,以回收缩合产物。为了进行比较,制备了不同的-SO3H官能化离子液体,并评估了它们在呋喃和糠醛缩合中的性能。发现具有烷基链接头的离子液体比咪唑鎓连接的N-磺酸更有效和酸性。除此之外,咪唑/三乙胺/吡啶和-SO3H之间的碳链长度增加,增加了相应离子液体的催化活性。在几种预离子化的液体中,强酸性咪唑基丁基磺酸(6)表现出最佳的活性,缩合产物的收率最高为84%。与传统的无机酸路线相比,该策略可显着提高呋喃和糠醛缩合产物的收率。-SO3H功能化的IL的活性和稳定性通过其成功的七个循环而得到证实,而没有丧失活性。然后,成功地扩展了这一新策略,使呋喃衍生物(例
-
[EN] MCT4 INHIBITORS FOR TREATING DISEASE<br/>[FR] INHIBITEURS DE MCT4 POUR LE TRAITEMENT DE MALADIES申请人:VETTORE LLC公开号:WO2016201426A1公开(公告)日:2016-12-15Disclosed herein are compounds and compositions useful in the treatment of MCT4 mediated diseases, such as proliferative and inflammatory diseases, having the structure of Formula I: Methods of inhibition MCT4 activity in a human or animal subject are also provided.
-
Cyclopentadienyliron dicarbonyl dimer: A simple tool for the hydrosilylation of aldehydes and ketones under air作者:Thais Cordeiro Jung、Gilles Argouarch、Pierre van de WegheDOI:10.1016/j.catcom.2016.01.033日期:2016.3The readily available iron complex [CpFe(CO)2]2 (1) exhibits good catalytic activity in the hydrosilylation of aldehydes and ketones in the presence of diethoxymethylsilane. The procedure described is air-tolerant and applicable to a wide range of substrates.
-
Ammonia borane enabled upgrading of biomass derivatives at room temperature作者:Wenfeng Zhao、Sebastian Meier、Song Yang、Anders RiisagerDOI:10.1039/d0gc02372h日期:——Simplifying biomass conversion to valuable products with high efficiency is pivotal for the sustainable development of society. Herein, an efficient catalyst-free system using ammonia borane (AB) as the hydrogen donor is described, which enables controllable reaction selectivity towards four value-added products in excellent yield (82–100%) under very mild conditions. In particular, the system is uniquely
-
Palladium-metalated porous organic polymers as recyclable catalysts for chemoselective decarbonylation of aldehydes作者:Wen-Hao Li、Cun-Yao Li、Yan Li、Hai-Tao Tang、Heng-Shan Wang、Ying-Ming Pan、Yun-Jie DingDOI:10.1039/c8cc03109f日期:——A novel palladium nanoparticle (NP)-metalated porous organic ligand (Pd NPs/POL-xantphos) has been prepared for the chemoselective decarbonylation of aldehydes. This heterogenous catalyst not only has excellent catalytic activity and chemoselectivity, but also holds high activity after 10 runs of reuse. The effective usage of this method is demonstrated through the synthesis of biofuels such as furfuryl
表征谱图
-
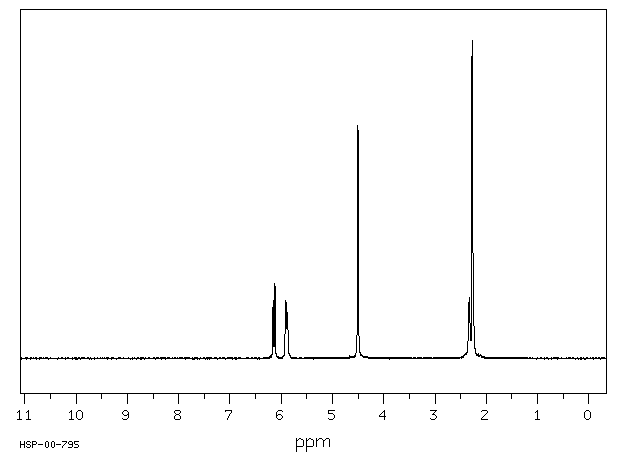
氢谱1HNMR
-
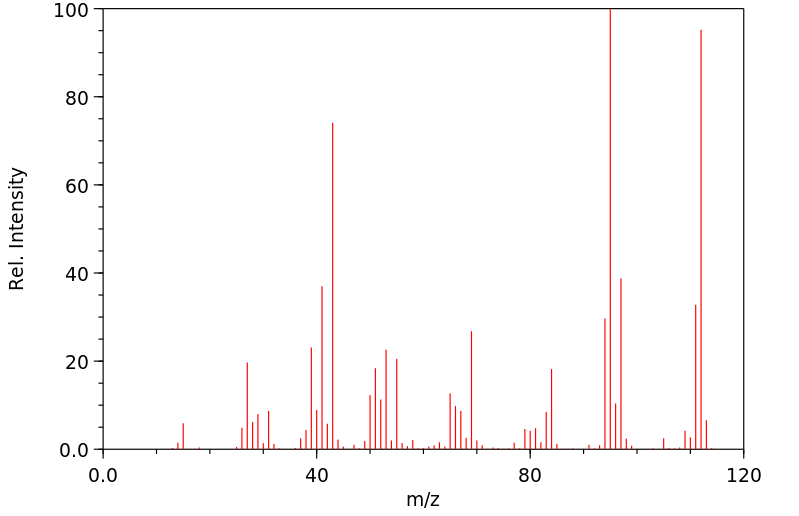
质谱MS
-
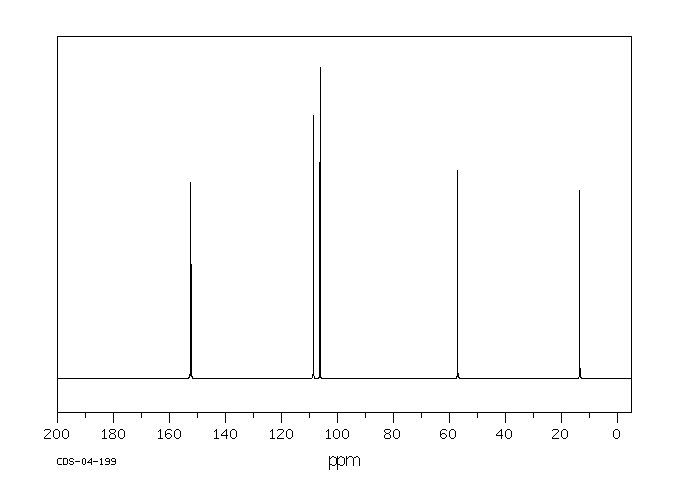
碳谱13CNMR
-
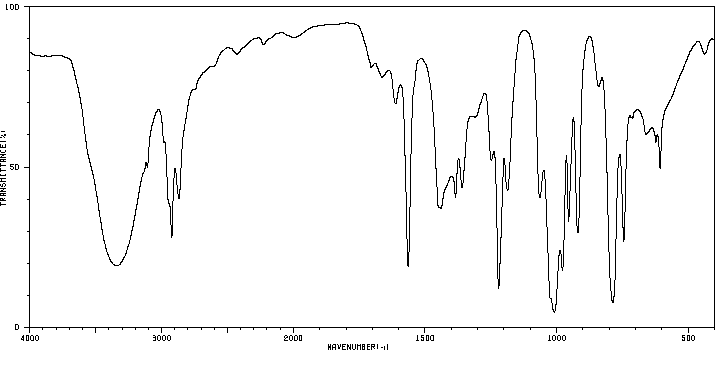
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香薷二醇
顺式-1-(2-呋喃基)-1-戊烯
顺-1,2-二氰基-1,2-双(2,4,5-三甲基-3-噻吩基)乙烯
顺-1,2-(2-噻嗯基)二乙烯
雷尼替丁-N,S-二氧化物
雷尼替丁-N-氧化物
钴(II)双[(2-吡啶基甲基)(叔丁基二甲基甲硅烷基)酰胺]
西拉诺德
螺[环氧乙烷-2,3'-吡咯并[1,2-a]吡嗪]
萘并[2,1,8-def]喹啉
苯硫基溴化镁
苯甲酸,2-[[[7-[[(3.β.)-3-羟基-28-羰基羽扇-20(29)-烯-28-基]amino]庚基]氨基]羰基]
苍术素
羟胺,O-[4-(2-呋喃基)丁基]-
缩水甘油糠醚
紫苏烯
糠醛肟
糠醛氰醇的1-乙氧基乙基醚
糠醇-d2
糠醇
糠基硫醇-d2
糠基硫醇
糠基甲基硫醚
糠基氯
糠基氨基甲酸异丙酯
糠基丙基醚
糠基丙基二硫醚
糠基3-巯基-2-甲基丙酸酯
糠基-异戊基醚
糠基-异丁基醚
糠基 2-甲基-3-呋喃基二硫醚
磷杂茂
碘化N,N,N-三甲基丁烷-1-铵
硫酸异丙基糠酯
硫代磷酸O-糠基O-甲基S-(2-丙炔基)酯
硫代磷酸O-乙基O-糠基S-(2-丙炔基)酯
硫代甲酸S-糠酯
硫代噻吩甲酰基三氟丙酮
硫代乙酸糠酯
硫代丙酸糠酯
硒吩-3-羧酸酰肼
硅烷,三(1-甲基乙基)[(3-甲基-2-呋喃基)氧代]-
硅烷,[2-(3-呋喃基)乙烯基]三甲基-,(E)-
硅烷,(1,1-二甲基乙基)(2-呋喃基甲氧基)二甲基-
砷杂苯
甲酸糠酯
甲氧亚胺基呋喃乙酸铵盐
甲基糠基醚
甲基糠基二硫
甲基呋喃-2-基甲基氨基甲酸酯