2-甲基-5-溴苯甲酸 | 79669-49-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:167-171°C
-
沸点:319.4±30.0 °C(Predicted)
-
密度:1.599±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
安全说明:S26
-
危险品运输编号:UN 2811 6.1/PG 3
-
海关编码:2916399090
-
危险品标志:Xn,Xi
-
危险类别码:R22
-
WGK Germany:3
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
: 5-Bromo-2-methylbenzoic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H7BrO2
分子式
: 215.04 g/mol
分子量
成分 浓度
5-Bromo-2-methylbenzoic acid
-
化学文摘编号(CAS No.) 79669-49-1
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 溴化氢气
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
充气保存
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 167 - 171 °C
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国(UN)规定的名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (5-Bromo-2-methylbenzoic acid)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (5-Bromo-2-methylbenzoic acid)
国际空运危规: Toxic solid, organic, n.o.s. (5-Bromo-2-methylbenzoic acid)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-5-溴苯甲酸甲酯 methyl 2-methyl-5-bromobenzoate 79669-50-4 C9H9BrO2 229.073 对甲苯甲酸 ortho-methylbenzoic acid 118-90-1 C8H8O2 136.15 3-溴-4-甲基苯甲酸 3-bromo-4-methylbenzoic acid 7697-26-9 C8H7BrO2 215.046 1-(5-溴-2-甲基苯基)乙酮 1-(5-bromo-2-methylphenyl)ethanone 90326-54-8 C9H9BrO 213.074 苯酞 2-benzofuran-1(3H)-one 87-41-2 C8H6O2 134.134 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-5-溴苯甲酸甲酯 methyl 2-methyl-5-bromobenzoate 79669-50-4 C9H9BrO2 229.073 5-溴-2-甲酰基苯甲酸甲酯 methyl 5-bromo-2-formylbenzoate 1016163-89-5 C9H7BrO3 243.057 5-溴-2-甲基苯甲酸乙酯 ethyl 5-bromo-2-methylbenzoate 359629-91-7 C10H11BrO2 243.1 6-溴-3H-异苯并呋喃-1-酮 6-bromophthalide 19477-73-7 C8H5BrO2 213.03 2-甲基-5-溴苯甲酸叔丁酯 5-bromo-2-methyl-benzoic acid tert-butyl ester 1202551-75-4 C12H15BrO2 271.154 2-甲基-3-溴苯甲酸甲酯 methyl 3-bromo-2-methylbenzoate 99548-54-6 C9H9BrO2 229.073 5-溴-2-溴甲苯甲酸甲酯 methyl 5-bromo-2-(bromomethyl)benzoate 79670-17-0 C9H8Br2O2 307.969 5-溴-2-甲基苯甲醇 (5-bromo-2-methylphenyl)methanol 258886-04-3 C8H9BrO 201.063 5-溴-2-甲基苯甲醛 5-bromo-2-methylbenzaldehyde 90050-59-2 C8H7BrO 199.047 —— ethyl 5-bromo-2-(bromomethyl)benzoate 950741-84-1 C10H10Br2O2 321.996 5-溴-2-(羧基甲基)苯甲酸 5-Bromo-2-carboxymethyl-benzoic acid 19725-82-7 C9H7BrO4 259.056 —— (5-bromo-2-bromomethylphenyl)methanol 1261620-07-8 C8H8Br2O 279.959 2-甲基-3-氨基-5-溴苯甲酸甲酯 methyl 3-amino-5-bromo-2-methylbenzoate 1000342-11-9 C9H10BrNO2 244.088 5-甲酰基-2-甲基苯甲酸 5-formyl-2-methylbenzoic acid 105650-34-8 C9H8O3 164.161 5-溴-2-甲氧基苄氯 5-bromo-2-methylbenzoyl chloride 21900-41-4 C8H6BrClO 233.492 1-(5-溴-2-甲基苯基)乙酮 1-(5-bromo-2-methylphenyl)ethanone 90326-54-8 C9H9BrO 213.074 5-溴-2-甲基苯甲酰胺 5-bromo-2-methylbenzamide 854633-21-9 C8H8BrNO 214.062 5-羟基-2-甲基苯甲酸 2-methyl-5-hydroxybenzoic acid 578-22-3 C8H8O3 152.15 - 1
- 2
反应信息
-
作为反应物:描述:2-甲基-5-溴苯甲酸 在 四(三苯基膦)钯 硫酸 、 benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate 、 水 、 硝酸 、 铁粉 、 sodium carbonate 、 氯化铵 、 溶剂黄146 、 sodium hydroxide 作用下, 以 1,4-二氧六环 、 1,1-二氯乙烷 、 乙醇 、 水 、 二甲基亚砜 、 N,N-二甲基甲酰胺 、 丙酮 、 乙腈 为溶剂, 反应 24.83h, 生成 N-[(1,2-二氢-4,6-二甲基-2-氧代-3-吡啶基)甲基]-5-[乙基(四氢-2H-吡喃-4-基)氨基]-4-甲基-4'-(4-吗啉基甲基)-[1,1'-联苯]-3-甲酰胺参考文献:名称:Aryl- or Heteroaryl-Substituted Benzene Compounds摘要:本发明涉及芳基或杂芳基取代的苯化合物。本发明还涉及含有这些化合物的药物组合物,以及通过向需要的受试者投与这些化合物和药物组合物来治疗癌症的方法。本发明还涉及利用这些化合物进行研究或其他非治疗目的的用途。公开号:US20120264734A1
-
作为产物:描述:参考文献:名称:含Co(II)槲皮素2,3-双加氧酶的ES(酶-底物)复合物的结构和功能模型系列摘要:一系列的单核的Co II -flavonolate络合物[CO II大号- [R(FLA)](大号ř ħ = 2 - {[双(吡啶-2-基甲基)氨基]甲基} - p /米-R -苯甲酸; R = p -OMe(1),p -Me(2),m -Br(4)和m -NO 2(5); fla =黄酮酸酯)被设计并合成为ES(酶-底物)复合物的结构和功能模型,以模拟含Co(II)的槲皮素2,3-二加氧酶(Co-2,3-QD)的活性位点。每个络合物中的金属中心Co(II)离子显示出相似的扭曲八面体几何形状。模型复合物在低温下显示出高的酶型双加氧反应性(配位底物黄酮酸酯的氧化O-杂环开环),这可能是由于配体中的羧酸酯基团所致。反应性表现出依赖于取代基的顺序-OMe(1)> -Me(2)> -H(3)14b> -Br(4)> -NO 2(5),而Hammett图是线性的(ρ= −0.78)。这可以解释为配体DOI:10.1021/ic402695c
文献信息
-
有害生物防除方法申请人:住友化学株式会社公开号:JP2021152084A公开(公告)日:2021-09-30【課題】有害生物の防除方法を提供すること。【解決手段】式(I)〔式(I)中、QはQ1で示される基(●はベンゼン環との結合部位を表す)等を表し、Eは、C1−C6鎖式炭化水素基等を表し、R1は、C1−C3鎖式炭化水素基等を表し、nは、0、1、2又は3を表し、nが2又は3である場合、複数のR2は同一又は異なっていてもよく、R2は、C1−C3鎖式炭化水素基等を表す。〕で示される化合物、又はそのNオキシド若しくはそれらの塩は有害生物を防除することができる。【選択図】なし
-
Silver-Catalyzed Oxidative Activation of Benzylic CH Bonds for the Synthesis of Difluoromethylated Arenes作者:Peng Xu、Shuo Guo、Liyan Wang、Pingping TangDOI:10.1002/anie.201400225日期:2014.6.2A mild and catalytic method to form difluoromethylated arenes through the activation of benzylic CH bonds has been developed. Utilizing AgNO3 as the catalyst, various arenes with diverse functional groups undergo activation/fluorination of benzylic CH bonds with commercially available Selectfluor reagent as a source of fluorine in aqueous solution. The reaction is operationally simple and amenable
-
[EN] HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS AND COMBINATIONS THEREOF<br/>[FR] MODULATEURS HÉTÉROCYCLIQUES DE LA SYNTHÈSE DES LIPIDES ET COMBINAISONS EN CONTENANT申请人:3 V BIOSCIENCES INC公开号:WO2015095767A1公开(公告)日:2015-06-25Heterocyclic modulators of lipid synthesis are provided as well as pharmaceutically acceptable salts thereof; pharmaceutical compositions comprising such compounds; and methods of treating conditions characterized by disregulation of a fatty acid synthase pathway by the administration of such compounds and combinations of such compounds and other therapeutic agents.提供了杂环调节剂脂质合成以及其药用盐;包括这些化合物的药物组合物;以及通过给予这些化合物和其他治疗剂的组合来治疗脂肪酸合酶途径失调症状的方法。
-
Design, synthesis and anticancer activity of N-(1-(4-(dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)-1-oxo-3-phenylpropan-2-yl derivatives作者:Mura Reddy Gudisela、N. Srinivasu、Chaitanya Mulakayala、Praveen Bommu、M.V. Basaveswara Rao、Naveen MulakayalaDOI:10.1016/j.bmcl.2017.07.029日期:2017.9Novel N-(1-(4-(dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)-1-oxo-3-phenylpropan-2-yl derivatives were designed, synthesized and their chemical structures were confirmed by 1H NMR, 13C NMR and Mass spectra. The anticancer activities of the newly synthesized compounds were evaluated in vitro against three human cancer cell lines including K562, Colo-205 and MDA-MB 231 by MTT assay. The screening
-
Synthesis and Broad Antiviral Activity of Novel 2-aryl-isoindolin-1-ones towards Diverse Enterovirus A71 Clinical Isolates作者:Yixuan Wang、Huiqiang Wang、Xinbei Jiang、Zhi Jiang、Tingting Guo、Xingyue Ji、Yanping Li、Yuhuan Li、Zhuorong LiDOI:10.3390/molecules24050985日期:——
Enterovirus 71 (EV-A71) is the main causative pathogen of childhood hand, foot and mouth disease. Effective medicine is currently unavailable for the treatment of this viral disease. Using the fragment-hopping strategy, a series of 2-aryl-isoindolin-1-one compounds were designed, synthesized and investigated for their in vitro antiviral activity towards multiple EV-A71 clinical isolates (H, BrCr, Shenzhen98, Jiangsu52) in Vero cell culture in this study. The structure–activity relationship (SAR) studies identified 2-phenyl-isoindolin-1-ones as a new potent chemotype with potent antiviral activity against EV-A71. Ten out of the 24 tested compounds showed significant antiviral activity (EC50 < 10 µM) towards four EV-A71 strains. Compounds A3 and A4 exhibited broad and potent antiviral activity with the 50% effective concentration (EC50) values in the range of 1.23–1.76 μM. Moreover, the selectivity indices of A3 and A4 were significantly higher than those of the reference compound, pirodavir. The western blotting experiment indicated that the viral VP1 was significantly decreased at both the protein and RNA level in a dose-dependent manner following treatment with compound A3. Moreover, compound A3 inhibited the viral replication by acting on the virus entry stage. In summary, this study led to the discovery of 2-aryl-isoindolin-1-ones as a promising scaffold with potent anti-EV-A71 activities, which deserves further in-depth studies.
肠道病毒71型(EV-A71)是儿童手足口病的主要病原体。目前还没有有效的药物治疗这种病毒性疾病。在本研究中,我们采用片段跳跃策略,设计、合成了一系列2-芳基-异吲哚啉-1-酮化合物,并研究了它们在Vero细胞培养中对多个EV-A71临床分离株(H、BrCr、深圳98、江苏52)的体外抗病毒活性。结构-活性关系(SAR)研究确定2-苯基-异吲哚啉-1-酮是一种对EV-A71具有强大抗病毒活性的新型有效化学型。在24个测试化合物中,有10个化合物对四种EV-A71毒株显示出显著的抗病毒活性(EC50<10µM)。化合物A3和A4表现出广泛而强大的抗病毒活性,50%有效浓度(EC50)值在1.23-1.76μM范围内。此外,A3和A4的选择性指数显著高于参照化合物皮罗达韦。western印迹实验表明,经化合物A3处理后,病毒的VP1在蛋白质和RNA水平上均显著降低,并呈剂量依赖性。此外,化合物A3通过作用于病毒进入阶段来抑制病毒复制。总的来说,本研究发现了2-芳基-异吲哚啉-1-酮作为一种具有强大抗EV-A71活性的有希望的结构,值得进一步深入研究。
表征谱图
-
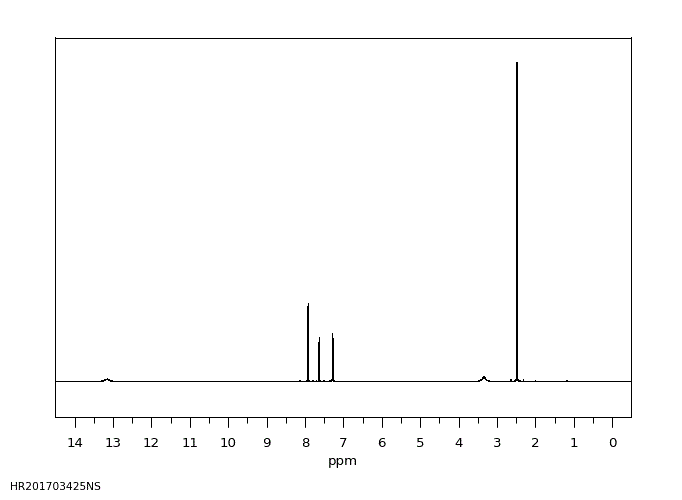
氢谱1HNMR
-
质谱MS
-
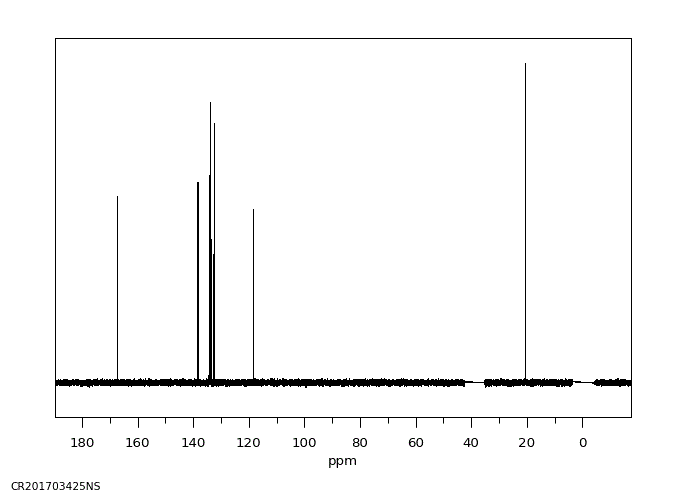
碳谱13CNMR
-
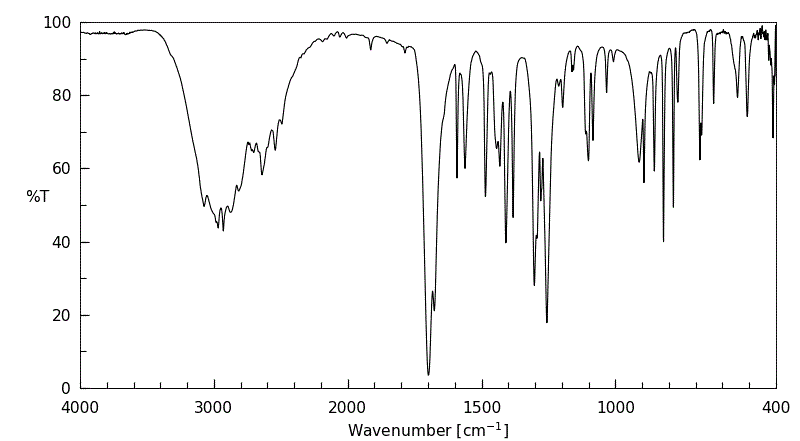
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息