5-溴-1H-苯并咪唑 | 4887-88-1
中文名称
5-溴-1H-苯并咪唑
中文别名
5-溴苯并咪唑;6-溴-1H-苯并咪唑;5-溴-1H-苯并[D]咪唑;1H-苯并咪唑,6-溴;6-溴-1H-苯并[d]咪唑
英文名称
5-bromo-1H-benzo[d]imidazole
英文别名
5-bromobenzimidazole;5-bromo-1H-benzimidazole;5-bromo-1H-benzoimidazole;6-bromo-1H-benzimidazole;5(6)-Brom-benzimidazol;5-Brom-benzimidazol
CAS
4887-88-1
化学式
C7H5BrN2
mdl
MFCD00160001
分子量
197.034
InChiKey
GEDVWGDBMPJNEV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:130 °C
-
沸点:417.4±18.0 °C(Predicted)
-
密度:1.770±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:28.7
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S22,S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2933990090
-
WGK Germany:3
-
危险标志:GHS07
-
危险性描述:H302
-
危险性防范说明:P301 + P312 + P330
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 5-Bromo-1H-benzo[d]imidazole
Synonyms: 5-Bromobenzimidazole
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Bromo-1H-benzo[d]imidazole
CAS number: 4887-88-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5BrN2
Molecular weight: 197.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 5-Bromo-1H-benzo[d]imidazole
Synonyms: 5-Bromobenzimidazole
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Bromo-1H-benzo[d]imidazole
CAS number: 4887-88-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H5BrN2
Molecular weight: 197.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-溴-1,3-二氢-2H-苯并咪唑-2-硫酮 5-bromo-1H-benzo[d]imidazole-2(3H)-thione 68468-39-3 C7H5BrN2S 229.1 苯并咪唑 benzoimidazole 51-17-2 C7H6N2 118.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-溴-1-甲基-1H-苯并[d]咪唑 5-bromo-1-methyl-1H-benzo[d]imidazole 53484-15-4 C8H7BrN2 211.061 6-溴-1-甲基-1H-苯并[D]咪唑 6-bromo-1-methyl-1H-benzo[d]imidazole 53484-16-5 C8H7BrN2 211.061 苯并咪唑 benzoimidazole 51-17-2 C7H6N2 118.138 5-溴-1-乙基苯并咪唑 5-bromo-1-ethyl-1H-benzimidazole 23073-51-0 C9H9BrN2 225.088 6-溴-1-乙基-1H-苯并咪唑 6-bromo-1-ethyl-1H-benzimidazole 813449-00-2 C9H9BrN2 225.088 5-溴-1-丙烷-2-基苯并咪唑 5-bromo-1-isopropyl-1H-benzo[d]imidazole 1200114-01-7 C10H11BrN2 239.115 2-(5-溴-1H-苯并[d]咪唑-1-基)乙醇 2-(5-bromo-1H-benzimidazol-1-yl)ethanol 116465-66-8 C9H9BrN2O 241.087 —— tert-butyl 5-bromo-1H-benzo[d]imidazole-2-carboxylate 1021919-13-0 C12H13BrN2O2 297.151
反应信息
-
作为反应物:描述:5-溴-1H-苯并咪唑 在 tris-(dibenzylideneacetone)dipalladium(0) 、 N,O-双三甲硅基乙酰胺 、 三氟甲磺酸三甲基硅酯 、 potassium acetate 、 2-二环己基磷-2,4,6-三异丙基联苯 作用下, 以 1,4-二氧六环 、 1,2-二氯乙烷 为溶剂, 反应 4.75h, 生成 [(2R,3R,4R,5R)-3,4-dibenzoyloxy-5-[6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzimidazol-1-yl]oxolan-2-yl]methyl benzoate参考文献:名称:A one pot three-step process for the synthesis of an array of arylated benzimidazoribosyl nucleosides摘要:开发了一种三步法一锅反应/纯化协议,以便快速获得基于苯并咪唑的核苷,其中酰基苯并咪唑核糖核苷与硼酸酯的衍生物作为关键反应中间体。DOI:10.1039/c0ob00866d
-
作为产物:描述:苯并咪唑 在 sulfonic acid functionalized silica N-溴代丁二酰亚胺(NBS) 作用下, 以 乙醚 、 乙腈 为溶剂, 反应 3.0h, 以77%的产率得到5-溴-1H-苯并咪唑参考文献:名称:An efficient, rapid and regioselective nuclear bromination of aromatics and heteroaromatics with NBS using sulfonic-acid-functionalized silica as a heterogeneous recyclable catalyst摘要:A simple, efficient and rapid method has been developed for high-yielding regioselective nuclear monobromination of aromatic and heteroaromatic compounds using NBS in the presence of sulfonic-acid-functionalized silica at room temperature. The catalyst works under heterogeneous conditions and can be recycled. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2006.10.029
文献信息
-
[EN] NOVEL SMALL MOLECULE INHIBITORS OF TEAD TRANSCRIPTION FACTORS<br/>[FR] NOUVEAUX INHIBITEURS À PETITES MOLÉCULES DE FACTEURS DE TRANSCRIPTION TEAD申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2020190774A1公开(公告)日:2020-09-24The present disclosure compounds, as well as their compositions and methods of use. The compounds inhibit the activity of the TEAD transcription factor, and are useful in the treatment of diseases related to the activity of TEAD transcription factor including, e.g., cancer and other diseases.
-
FLAP MODULATORS申请人:JANSSEN PHARMACEUTICA NV公开号:US20150259357A1公开(公告)日:2015-09-17The present invention relates to compounds of Formula (I), or a form thereof, wherein ring A, R 1 , R 2 , R 3 , R 3 ′, L, W, and V are as defined herein, useful as FLAP modulators. The invention also relates to pharmaceutical compositions comprising compounds of Formula (I). Methods of making and using the compounds of Formula (I) are also within the scope of the invention本发明涉及式(I)的化合物,或其形式,其中环A,R1,R2,R3,R3',L,W和V如本文所定义,可用作FLAP调节剂。该发明还涉及包含式(I)化合物的药物组合物。制备和使用式(I)化合物的方法也属于本发明的范围。
-
4-AZETIDINYL-1-PHENYL-CYCLOHEXANE ANTAGONISTS OF CCR2申请人:Zhang Xuqing公开号:US20100267689A1公开(公告)日:2010-10-21The present invention comprises compounds of Formula (I): wherein: X, R 1 , R 2 , R 3 , and R 4 are as defined in the specification. The invention also comprises a method of preventing, treating or ameliorating a syndrome, disorder or disease, wherein said syndrome, disorder or disease is type II diabetes, obesity and asthma. The invention also comprises a method of inhibiting CCR2 activity in a mammal by administration of a therapeutically effective amount of at least one compound of Formula (I).本发明涵盖了以下式(I)的化合物: 其中:X,R1,R2,R3和R4如规范中所定义。该发明还涵盖了一种预防、治疗或改善综合征、疾病或疾病的方法,其中所述综合征、疾病或疾病是II型糖尿病、肥胖和哮喘。该发明还涵盖了通过给哺乳动物施用至少一种式(I)化合物的治疗有效量来抑制CCR2活性的方法。
-
Endogenous X–CO species enable catalyst-free formylation prerequisite for CO<sub>2</sub> reductive upgrading作者:Hongguo Wu、Wenshuai Dai、Shunmugavel Saravanamurugan、Hu Li、Song YangDOI:10.1039/d0gc02142c日期:——(>90%) from catalyst-free reductive upgrading of CO2 under mild conditions (50 °C). The endogenous X–CO species, derived from the N-methyl-substituted amide-based solvent [Me2N–C(O)–R], especially PolarClean, and O-formyl group [O–C(O)–H] of in situ formed silyl formate, were found to play a prominent promotional role in the activation of the used hydrosilane for reductive CO2 insertion, as demonstratedCO 2是温室气体的主要成分,目前已被开发为一种有前途的碳原料替代品。在各种转化途径中,经历催化还原的CO 2可以提供氢/能量载体和增值化学品,而特定的含金属催化剂或有机催化剂通常是顺利进行所涉及的反应过程的先决条件。在这项工作中,可以在温和的条件下通过无催化剂的CO 2还原提质,以高收率(> 90%)合成甲酸和含N的苯并杂环化合物(包括各种苯并咪唑,苯并噻唑和苯并恶唑)以及硅烷醇。50°C)。来自N的内源X–C O物种甲基取代的酰胺基溶剂[Me 2 N–C(O)–R],尤其是PolarClean,以及原位形成的甲硅烷基甲酸酯的O-甲酰基[ OC – O (H)–H]如密度泛函理论(DFT)计算和同位素标记实验所证明的那样,在用于还原的CO 2插入的已用氢硅烷的活化中,显着的促进作用。此外,还描述了反应机理和基于条件的敏感性评估。
-
Convenient Two-step One-pot Synthesis of Benzimidazoles Using 2-nitroanilines and Thiourea Dioxide作者:Shuai Pu、Qiuxiang Liang、Xi Luo、Juan LuoDOI:10.3184/174751914x13896361235211日期:2014.2
A new convenient method for the conversion of 2-nitroanilines into benzimidazoles by two-step one-pot procedure is reported. The procedure involves the reduction of nitro group followed by the intramolecular cyclisation of the corresponding o-phenylenediamines utilising thiourea dioxide and sodium hydroxide at 70 °C in mixed solvents of H2O and EtOH (v/v, 3/1). The yields are good to excellent and the workup is simple.
表征谱图
-
氢谱1HNMR
-
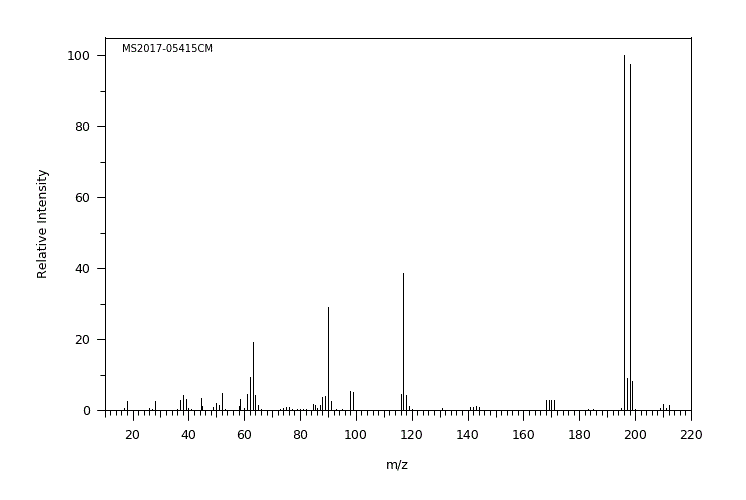
质谱MS
-
碳谱13CNMR
-
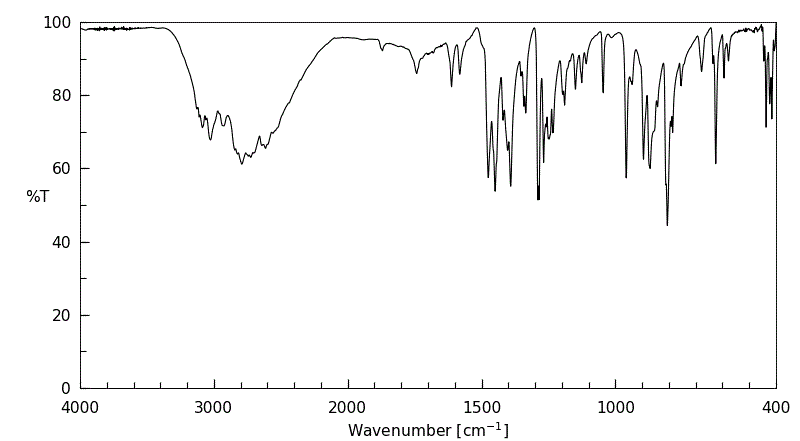
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10