溴脲苷 | 59-14-3
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:191-194 °C (dec.)(lit.)
-
比旋光度:31 º (c=1, 0.1N NaOH)
-
密度:1.8573 (rough estimate)
-
溶解度:NH4OH:0.1Mat20℃C,透明,无色
-
LogP:-0.290
-
物理描述:5-bromo-2'-deoxyuridine is a white crystalline powder. (NTP, 1992)
-
颜色/状态:Crystals from absolute ethanol
-
蒸汽压力:1.7X10-14 mm Hg at 25 °C (est)
-
稳定性/保质期:
Solid BrdU is stable in the dark to heat and humidity for 3 months at temperatures below 60 °C. On exposure to sunlight there is discoloration to grayish-brown. Ultraviolet irritations of frozen aqueous solutions of BrdU results in liberation of bromide ion and formation of a debrominated dimer, while radiolysis of aqueous solutions in the presence of oxygen yields mainly bromide, isodialuric acid deoxyriboside, and smaller amounts of various oxygenated derivatives.
-
旋光度:(c = 1, 0.1 N NaOH) [a]20 D = +31 ± 1°
-
分解:When heated to decomposition it emits very toxic fumes of /hydrogen bromide and nitrogen oxides/.
-
解离常数:pKa = 7.27 (imide nitrogen) (est)
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:99.1
-
氢给体数:3
-
氢受体数:5
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S26,S36/37/39
-
危险类别码:R68,R36/37/38
-
WGK Germany:2
-
海关编码:29349990
-
危险品运输编号:OTH
-
RTECS号:YU7350000
-
危险标志:GHS08
-
危险性描述:H340,H361
-
危险性防范说明:P201,P281,P308 + P313
SDS
制备方法与用途
Bromodeoxyuridine(BrdU,5-Bromo-2'-deoxyuridine,BUdR)是一种核苷类似物,能够与胸腺嘧啶竞争性地整合到DNA中,用于检测增生细胞。
体外研究在RG2大鼠胶质瘤细胞中,Bromodeoxyuridine表现出对癌细胞系和癌症干细胞数量扩增的诱导作用,并且剂量依赖性地抑制了其增殖。在H9细胞和BJ纤维母细胞中,Bromodeoxyuridine引起了细胞周期分布的变化。由于BrdU能够稳定整合到DNA中,因此可用于评估细胞增殖及其他相关细胞过程。
体内研究在大鼠胶质瘤RG2肿瘤模型中,通过腹腔注射(300 mg/kg)或口服给予(0.8 mg/ml),Bromodeoxyuridine显著减缓了肿瘤的形成。
化学性质Bromodeoxyuridine是一种无臭、无味的白色结晶性粉末。它难溶于水和甲醇,几乎不溶于氯仿和苯,但易溶于氢氧化钠溶液。熔点为193-197℃(分解),比旋度+23°(C=1,水中)。
用途Bromodeoxyuridine作为脑瘤和头颈部肿瘤的放射增敏剂,能够提高放疗的效果。与其他抗代谢剂联合使用也能增强治疗效果。
类别有毒物质
毒性分级低毒
急性毒性- 大鼠口服LD50:8400毫克/公斤
- 小鼠口服LD50:9100毫克/公斤
热分解会释放有毒的氮氧化物和溴化物烟雾
储运特性应存放在通风、低温、干燥的地方
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二-o-乙酰基-5-溴-2-脱氧-d-尿苷 5-bromo-3',5'-di-O-acetyl-2'-deoxyuridine 6161-23-5 C13H15BrN2O7 391.175 2-脱氧尿苷 2'-Deoxyuridine 951-78-0 C9H12N2O5 228.205 beta-胸苷 thymidine 146183-25-7 C10H14N2O5 242.232 二乙酰基-2'脱氧尿苷 3',5'-di-O-acetyl-2'-deoxyuridine 13030-62-1 C13H16N2O7 312.279 碘苷 5-Iodo-2'-deoxyuridine 54-42-2 C9H11IN2O5 354.101 5-(三甲基锡烷基)-2'-脱氧尿苷 5-trimethylstannyl-2'-deoxyuridine 146629-34-7 C12H20N2O5Sn 391.011 TAS-102杂质 5-carboxy-2'-deoxyuridine 14599-46-3 C10H12N2O7 272.214 2'-脱氧胞嘧啶核苷 2'-Deoxycytidine 951-77-9 C9H13N3O4 227.22 5-甲基尿甙 5-Methyluridine 1463-10-1 C10H14N2O6 258.231 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(5-bromouracil-1-yl)-1,2-dideoxy-β-D-erythro-pentofuranuronic acid 3180-31-2 C9H9BrN2O6 321.084 5-溴-2'-脱氧尿苷 5'-单磷酸酯 5Br-dUMP 6666-38-2 C9H12BrN2O8P 387.081 —— 5'-O-lauroyl-5-bromo-2'-deoxyuridine 1111240-17-5 C21H33BrN2O6 489.407 —— 5-bromo-5'-<(isobutyloxy)carbonyl>-2'-deoxyuridine 130328-04-0 C14H19BrN2O7 407.218 3,5-二-o-乙酰基-5-溴-2-脱氧-d-尿苷 5-bromo-3',5'-di-O-acetyl-2'-deoxyuridine 6161-23-5 C13H15BrN2O7 391.175 —— 3',5'-O-bis-(trimethylsilyl)-5-bromo-2'-deoxyuridine 34279-86-2 C15H27BrN2O5Si2 451.465 —— 5-Bromo-2'-deoxy-5'-O-(phosphonoacetyl)uridine 117627-07-3 C11H14BrN2O9P 429.118 2-脱氧尿苷 2'-Deoxyuridine 951-78-0 C9H12N2O5 228.205 —— 3',5'-O-bis-(tetrahydropyranyl)-5-bromo-2'-deoxyuridine 93182-32-2 C19H27BrN2O7 475.337 —— 5-Bromo-2'-deoxy-3'-O-(phosphonoacetyl)uridine 117627-08-4 C11H14BrN2O9P 429.118 5-氨基-2'-脱氧尿苷 5-amino-2'-deoxyuridine 5536-30-1 C9H13N3O5 243.219 5-羟基-2-脱氧尿苷 5-hydroxy-2'-deoxyuridine 5168-36-5 C9H12N2O6 244.204 5'-O-[二(4-甲氧基苯基)苯基甲基]-5-溴-2'-脱氧尿苷 5-bromo-5'-O-(4,4'-dimethoxytrityl)-2'-deoxyuridine 63660-21-9 C30H29BrN2O7 609.473 beta-胸苷 thymidine 146183-25-7 C10H14N2O5 242.232 —— 5-bromo-3'-(4-methoxytrityl)-2'-deoxyuridine 130328-06-2 C29H27BrN2O6 579.447 —— 5-bromo-3′,5′-diacetyl-2′-deoxyuridine —— C13H15BrN2O7 391.175 —— 5-azido-2′-deoxyuridine 137359-50-3 C9H11N5O5 269.217 1-(2'-去氧基-alpha-D-呋喃核糖基)-5-三甲基硅烷基尿嘧啶 5-Trimethylsilyl-2'-deoxy-β-uridine 70523-31-8 C12H20N2O5Si 300.387 —— 3',5'-O-di(tert-butyldimethylsilyl)-2'-deoxyuridine 64911-18-8 C21H40N2O5Si2 456.73 —— 5-benzylamino-2'-deoxyuridine 1239968-06-9 C16H19N3O5 333.344 5-苯基硫代-2'-脱氧尿苷 5-phenylsulfanyl-2'-deoxyuridine 100634-97-7 C15H16N2O5S 336.368 —— 3',5'-bis-O-(trimethylsilyl)-thymidine 10457-18-8 C16H30N2O5Si2 386.596 —— 3,5-di-O-(tetrahydropyran-2-yl)thymidine 76541-05-4 C20H30N2O7 410.467 —— 5-amino-3',5'-di-O-(t-butyldimethylsilyl)-2'-deoxyuridine 145125-16-2 C21H41N3O5Si2 471.745 3,5-双-o-(t-丁基二甲基甲硅烷基)胸腺嘧啶脱氧核苷 3',5'-O-di(tert-butyldimethylsilyl)thymidine 40733-26-4 C22H42N2O5Si2 470.757 —— 5-bromo-(N4-2-hydroxyphenyl)-2'-deoxycytidine —— C15H16BrN3O5 398.213 —— 5-bromo-2'-deoxy-6,5'-cyclo-(β-D-ribofuranosyl)uridine 111476-27-8 C9H9BrN2O4 289.085 —— 1-(2-deoxy-3,5-di-O-t-butyldimethylsilyl-β-D-ribofuranosyl)imidazolin-2-one-4-carboxaldehyde —— C9H12N2O5 228.205 —— 1-(2-deoxy-β-D-ribofuranosyl)-2-oxo-2,3-dihydro-1H-imidazole-4-carboxylic acid 20406-83-1 C9H12N2O6 244.204 —— 1-(2-deoxy-α-D-ribofuranosyl)-2-oxo-2,3-dihydro-1H-imidazole-4-carboxylic acid —— C9H12N2O6 244.204 —— 1-(2-deoxy-β-D-ribofuranosyl)-2-oxo-2,3-dihydro-1H-imidazole-4-carboxamide 105386-22-9 C9H13N3O5 243.219 —— 1,3-diaza-2-oxophenoxazine 2'-deoxy-β-D-ribofuranoside —— C15H15N3O5 317.301 —— 3-((2R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)-methyl)-4-hydroxytetrahydrofuran-2-yl)-3,9-dihydro-2H-pyrimido-[4,5-b]indol-2-one 169243-52-1 C36H33N3O6 603.675 —— 3-((2R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxytetrahydrofuran-2-yl)benzo[b]pyrimido[4,5-e][1,4]oxazin-2(10H)-one 169243-58-7 C36H33N3O7 619.674 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:溴脲苷 在 platinum(IV) oxide 氧气 作用下, 以 水 为溶剂, 反应 8.0h, 以85%的产率得到1-(5-bromouracil-1-yl)-1,2-dideoxy-β-D-erythro-pentofuranuronic acid参考文献:名称:Synthesis of a Carboxamide Linked UBr∗UBrDimer–Duplex and Triplex Stabilities of the Corresponding Oligodeoxynucleotides摘要:A U-Br*U-Br dimer was synthesized by connecting the appropiate nucleoside monomers through a 5-atom carboxamide linkage. The dimer was incorporated in oligodeoxynucleotides and investigated for hybridization properties toward single and double stranded DNA.DOI:10.1080/15257779508010721
-
作为产物:参考文献:名称:5-取代的2'-脱氧尿苷异头体的立体选择性合成摘要:研究了5-取代的2,4-双(三甲基甲硅烷氧基)嘧啶与3,5-双(Op-氯苯甲酰基)-2-脱氧-α-D-呋喃核糖基氯的取代反应。在对硝基苯酚的存在下,β端基异构体立体选择性地形成,而有机碱的加入导致α端基异构体的立体选择性形成。反应的立体选择性取决于二甲硅烷基嘧啶的 5 位取代基、添加剂和每种试剂的浓度。5-取代的2'-脱氧-尿苷的α和β异头通过5-取代3',5'-二-O-(对-氯苯甲酰基)-2'-脱氧尿苷的α和β异头脱酰化合成。DOI:10.1246/bcsj.60.2073
-
作为试剂:描述:1-戊硫醇 、 12-Chloro-8,9,10,11-tetrahydro-7H-benzo[4,5]imidazo[1,2-a]cyclohepta[d]pyridine-6-carbonitrile 在 溴脲苷 作用下, 以 苯 为溶剂, 反应 4.0h, 以98%的产率得到12-Pentylsulfanyl-8,9,10,11-tetrahydro-7H-benzo[4,5]imidazo[1,2-a]cyclohepta[d]pyridine-6-carbonitrile参考文献:名称:Russell, Ronald K.; Nievelt, Caroline E. van, Journal of Heterocyclic Chemistry, 1995, vol. 32, # 1, p. 299 - 306摘要:DOI:
文献信息
-
[EN] PYRROLOPYRIMIDINES<br/>[FR] PYRROLOPYRIMIDINES申请人:JANSSEN PHARMACEUTICA NV公开号:WO2009016132A1公开(公告)日:2009-02-05The present invention relates to compounds or pharmaceutically-acceptable salts thereof, processes for preparing them, pharmaceutical compositions containing them and their use in therapy. The invention particularly relates to compounds that are polo-like kinase (PLKs) inhibitors useful for the treatment of disease states mediated by PLK, especially PLK4, in particular such compounds that are useful in the treatment of pathological processes which involve an aberrant cellular proliferation, such as tumour growth, rheumatoid arthritis, restenosis and atherosclerosis.本发明涉及化合物或其药用盐,制备它们的方法,含有它们的药物组合物以及它们在治疗中的用途。该发明特别涉及一类极化样激酶(PLKs)抑制剂化合物,用于治疗由PLK介导的疾病状态,特别是PLK4,特别是在治疗涉及异常细胞增殖的病理过程中有用的化合物,如肿瘤生长、类风湿性关节炎、再狭窄和动脉粥样硬化。
-
[EN] AZOLE COMPOUNDS AS PIM INHIBITORS<br/>[FR] COMPOSÉS D'AZOLE UTILISÉS EN TANT QU'INHIBITEURS DES PIM申请人:AMGEN INC公开号:WO2012129338A1公开(公告)日:2012-09-27The invention relates to bicyclic compounds of formulas I and Ia, and salts thereof. In some embodiments, the invention relates to inhibitors or modulators of Pim-1 and/or Pim-2, and/or Pim-3 protein kinase activity or enzyme function. In still further embodiments, the invention relates to pharmaceutical compositions comprising compounds disclosed herein, and their use in the prevention and treatment of Pim kinase related conditions and diseases, preferably cancer.
-
ANTI-B7-H3 ANTIBODIES AND ANTIBODY DRUG CONJUGATES
-
[EN] MACROCYLIC PYRIDINE DERIVATIVES<br/>[FR] DÉRIVÉS DE PYRIDINE MACROCYCLIQUES申请人:JANSSEN PHARMACEUTICA NV公开号:WO2015150557A1公开(公告)日:2015-10-08The present invention relates to substituted macrocylic pyrimidine derivatives of Formula (I) wherein the variables have the meaning defined in the claims. The compounds according to the present invention have EF2K inhibitory activity and optionally also Vps34 inhibitory activity. The invention further relates to processes for preparing such novel compounds, pharmaceutical compositions comprising said compounds as an active ingredient as well as the use of said compounds as a medicament.
-
[EN] COMPOUNDS COMPRISING CLEAVABLE LINKER AND USES THEREOF<br/>[FR] COMPOSÉS COMPRENANT UN LIEUR CLIVABLE ET LEURS UTILISATIONS申请人:INTOCELL INC公开号:WO2019008441A1公开(公告)日:2019-01-10Provided are a compound including a cleavable linker, a use thereof, and an intermediate compound for preparing the same, and more particularly, the compound including a cleavable linker of the present invention may include an active agent (for example, a drug, a toxin, a ligand, a probe for detection, etc.) having a specific function or activity, a SO2 functional group which is capable of selectively releasing the active agent, and a functional group which triggers a chemical reaction, a physicochemical reaction and/or a biological reaction by external stimulation, and may further include a ligand (for example, oligopeptide, polypeptide, antibody, etc.) having binding specificity for a desired target receptor.
表征谱图
-
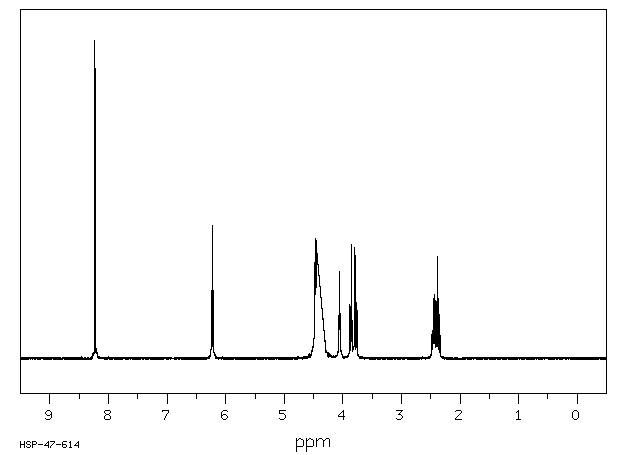
氢谱1HNMR
-
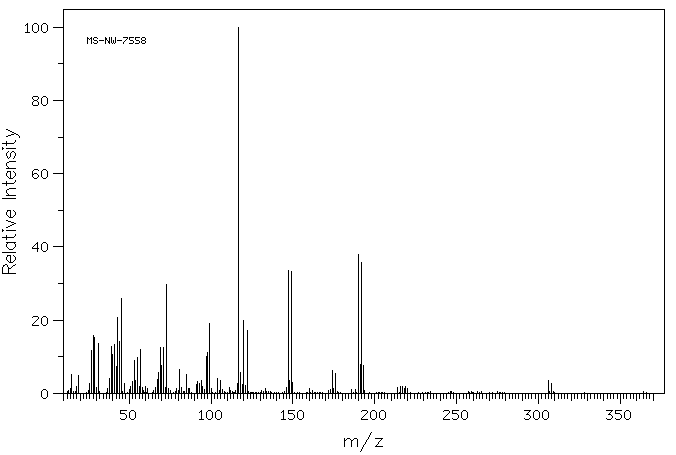
质谱MS
-
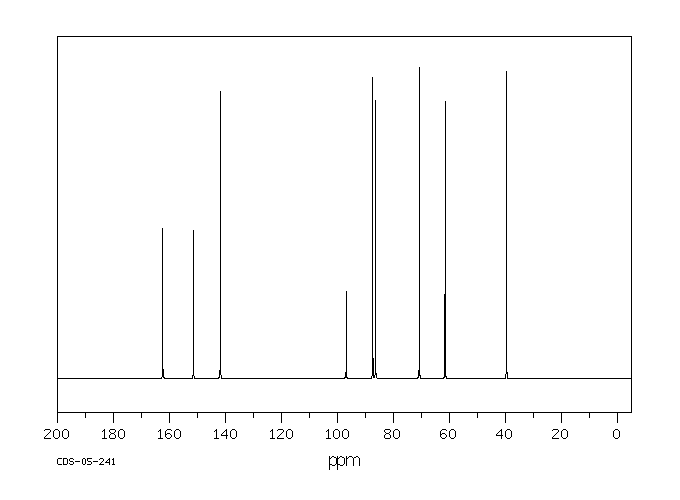
碳谱13CNMR
-
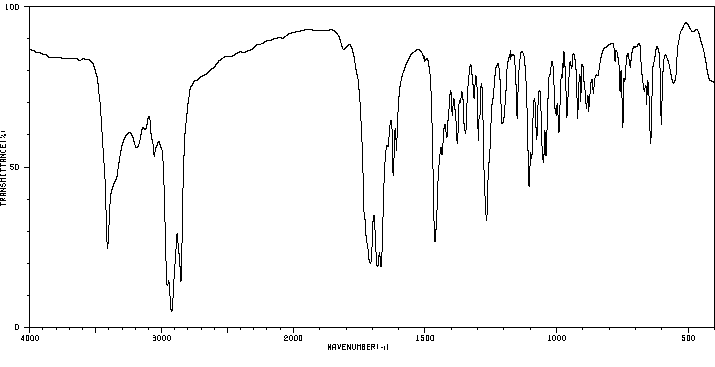
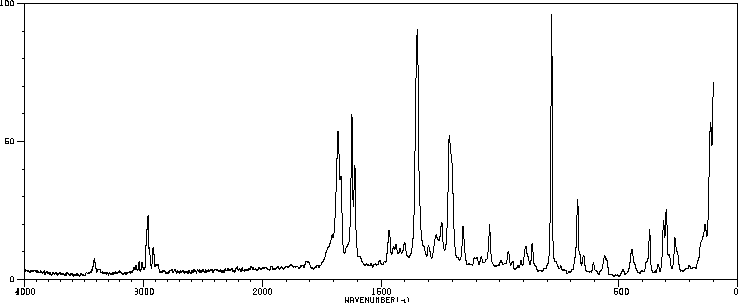
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息