甲苯-2,4-二异氰酸酯 | 584-84-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:20-22 °C(lit.)
-
沸点:124-126 °C18 mm Hg(lit.)
-
密度:1.225 g/mL at 25 °C
-
蒸气密度:6 (vs air)
-
闪点:250 °F
-
溶解度:与乙醚、丙酮、苯、四氯化碳和氯苯混溶。
-
暴露限值:TLV-TWA 0.0355 mg/m3 (0.005 ppm) (ACGIH and NIOSH); STEL or ceiling/10 min 0.142 mg/m3 (0.02 ppm) (ACGIH, NIOSH, and OSHA); IDLH 71 mg/m3 (10 ppm) (NIOSH).
-
LogP:3.43 at 22℃
-
物理描述:Toluene-2,4-diisocyanate appears as colorless to yellow or dark liquid or solid with a sweet, fruity, pungent odor. Melting point 68°F (20° C). Evolves CO2 when moist. This can cause over-pressurization in an enclosed space.Toxic and carcinogenic. Used in polyurethane foams, coatings in floor and wood finishes, sealers, paints, concrete sealers for aircraft and tank trucks, elastomers in clay pipe seals, elastomers and coatings, and crosslinking agent for nylon. Rate of onset: Immediate Persistence: Hours - weeks Odor threshold: 0.4 - 2 ppm Source/use/other hazard: Polyurethane (wood coatings, foam), nylon industries; skin irritant.
-
颜色/状态:Colorless to pale yellow, solid or liquid (above 71 °F)
-
气味:Sharp, pungent
-
蒸汽密度:6 (EPA, 1998) (Relative to Air)
-
蒸汽压力:VP: 0.5 mm Hg at 25 °C /80% 2,4:20% 2,6/
-
稳定性/保质期:
Darkens on exposure to sunlight.
-
自燃温度:620 °C (1148 °F)
-
分解:When heated to decomposition, it emits highly toxic fumes of ... /nitrogen oxides/.
-
燃烧热:-10300 Btu/lb = -5720 cal/g = -239x10+5 J/kg (est)
-
表面张力:25 dynes/cm = 0.025 N/m at 25 °C (est)
-
聚合:Polymerization: Slow, not hazardous, above 113 °F; Liquid-water interfacial tension: (est) 45 dynes/cm= 0.045 N/m at 25 °C
-
气味阈值:Odor Threshold Low: 0.05 [mmHg]; Odor Threshold High: 2.4 [mmHg]; Odor threshold from CHEMINFO; [ICSC] Odor threshold = 50 ppb with about 1/2 of volunteers detecting and identifying the material at 400 ppb
-
折光率:Index of refraction: 1.5654 at 25 °C/D
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:58.9
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:2.5 ppm
-
危险品标志:T+
-
安全说明:S23,S36/37,S45,S61
-
危险类别码:R52/53,R40,R36/37/38,R42/43,R26
-
WGK Germany:2
-
海关编码:29291010
-
危险品运输编号:UN 2078 6.1/PG 2
-
危险类别:6.1
-
RTECS号:CZ6300000
-
包装等级:II
-
危险标志:GHS06,GHS08
-
危险性描述:H315,H317,H319,H330,H334,H335,H351,H412
-
危险性防范说明:P260,P280,P284,P304 + P340 + P310,P342 + P311,P403 + P233
SDS
制备方法与用途
甲苯-2,4-二异氰酸酯是一种常温常压下无色透明的液体状化合物,对水分较为敏感。它虽然相对稳定,但容易水解,应存放在阴凉、干燥和通风良好的地方,避免阳光直射。
该物质是一种重要的有机合成中间体,具有较高的反应活性,能够与胺、醇和酚等进行反应,生成相应的脲、尿酸酯和酯类化合物。
甲苯-2,4-二异氰酸酯为无色液体。其熔点为19.5至21.5℃,沸点在251℃或于126℃(1.47kPa)下挥发;相对密度为1.2244(20/4℃),闪点为132℃。它能与乙醚、丙酮、四氯化碳、苯、氯苯和汽油混溶,并且与水和醇反应会分解。
商品简称TDI,主要由2,4-异构体和2,6-异构体混合而成,常见的牌号有TDI-65/35(2,4-异构体占65%)和TDI-80/20。前者熔点为3.5至5.5℃,后者为11.5至13.5℃。
甲苯-2,4-二异氰酸酯主要用于合成聚氨酯产品,包括泡沫塑料、聚氨酯涂料、聚氨酯橡胶;同时也应用于聚酰亚胺纤维和胶粘剂等。
该化合物用于纤维、泡沫塑料及橡胶的合成,并可用作粘合剂。其生产方法是通过将甲苯硝化生成二硝基甲苯,再经还原得到甲苯二胺。随后,甲苯二胺与光气反应即得TDI(以2,4-异构体为主)。
甲苯-2,4-二异氰酸酯被归类为有毒物品,属于剧毒物质。急性吸入毒性数据如下:大鼠LC50为98毫克/立方米/4小时;小鼠LC50为70毫克/立方米/4小时。皮肤刺激表现为兔子接触500毫克24小时后中度刺激;眼睛刺激则在兔子接触100毫克后表现出重度反应。
该物质遇空气混合可形成爆炸性气体,遇明火或高温会分解产生有毒氮氧化物气体。储存和运输时应注意通风干燥、低温,并与氧化剂、食品添加剂、酸类及碱类分开存放。
灭火方式建议使用泡沫、雾状水、二氧化碳、砂土或干粉;禁止使用酸碱灭火剂。
职业暴露标准为TLV-TWA 0.005 PPM(0.036毫克/立方米)和STEL 0.02 PPM(0.14 毫克/立方米),最大接触限值为0.02 PPM(0.14 毫克/ 立方米)。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲苯2,6-二异氰酸酯 2,6-Toluene diisocyanate 91-08-7 C9H6N2O2 174.159 间苯二异氰酸酯 1,3-Phenylene diisocyanate 123-61-5 C8H4N2O2 160.132 2,4-二氨基甲苯 2,4-Diaminotoluene 95-80-7 C7H10N2 122.17 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲苯2,6-二异氰酸酯 2,6-Toluene diisocyanate 91-08-7 C9H6N2O2 174.159 4-氯甲基-1,3-苯二异氰酸酯 1-(chloromethyl)-2,4-diisocyanatobenzene 51979-57-8 C9H5ClN2O2 208.604 —— 1-(Bromomethyl)-2,4-diisocyanatobenzene 115263-42-8 C9H5BrN2O2 253.055 甲苯-2,4-二异硫氰酸 1-methylbenzene-2,4-diisothiocyanate 4891-66-1 C9H6N2S2 206.292 —— 6-bromo-4-methyl-1,3-phenylene diisocyanate 55206-98-9 C9H5BrN2O2 253.055 N,N'-二(3-异氰酸-4-甲基苯基)脲 N,N'-bis-(3-isocyanato-4-methyl-phenyl)-urea 5206-52-0 C17H14N4O3 322.323 —— (3-isocyanato-4-methyl-phenyl)-carbamic acid methyl ester 91485-85-7 C10H10N2O3 206.201 2,4-二氨基甲苯 2,4-Diaminotoluene 95-80-7 C7H10N2 122.17 —— (3-Isocyanato-4-methyl-phenyl)-carbamic acid allyl ester 3205-02-5 C12H12N2O3 232.239 —— 3-isocyanato-4-methylcarbanilic acid tert-butyl ester 98096-49-2 C13H16N2O3 248.282 —— 1-(4-Butylphenyl)-3-(3-isocyanato-4-methylphenyl)urea 531534-05-1 C19H21N3O2 323.395 —— (3-isocyanato-4-methyl-phenyl)-carbamic acid butyl ester 100616-00-0 C13H16N2O3 248.282 —— 4-[(3-isocyanato-4-methylphenyl)carbamoyloxy]butyl N-(3-isocyanato-4-methylphenyl)carbamate —— C22H22N4O6 438.44 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Lussy, Chemische Berichte, 1875, vol. 8, p. 293摘要:DOI:
-
作为产物:描述:参考文献:名称:Reaction method accompanied by production of gas component摘要:本发明涉及一种反应方法,包括以下步骤:将含有至少一种原料化合物和一个标准沸点低于原料化合物的标准沸点的低沸点化合物的液体输送到流通道中;加热液体以通过原料化合物的反应生成液体反应产物和气体组分;将含有反应产物的液相与含有气体组分和低沸点化合物的气相分离。公开号:US10118110B2
-
作为试剂:描述:L-苯丙氨酸乙酯盐酸盐 在 lithium aluminium tetrahydride 、 三乙胺 、 N,N'-羰基二咪唑 、 甲苯-2,4-二异氰酸酯 作用下, 以 四氢呋喃 为溶剂, 反应 41.5h, 生成 (3S,11S,15S,23S)-3,11,15,23-Tetrabenzyl-1,7,13,19-tetraoxa-4,10,16,22-tetraaza-cyclotetracosane-2,5,9,12,17,21-hexaone参考文献:名称:由l-氨基酸合成的大环化合物及其通过有机液膜对氨基酯盐的选择性转运摘要:18-元大环的合成,24元大环化合物,和30元大环化合物,,,,,通过使用二甘醇酸和氨基酸作为构成成分来实现。氨基酯盐通过有机液体膜的选择性转运是由其大环和中间体化合物介导的。这些含有氨基酸部分的载体的转移能力不同于18-冠-6。DOI:10.1016/s0040-4020(01)86893-9
文献信息
-
SILANE COUPLING COMPOUNDS AND MEDICAL AND/OR DENTAL CURABLE COMPOSITIONS COMPRISING THE SAME申请人:KABUSHIKI KAISHA SHOFU公开号:US20190300552A1公开(公告)日:2019-10-03The present invention relate to a novel silane coupling agent and a medical and/or dental curable composition comprising the same. It is an object of the present invention to provide a novel silane coupling agent that imparts high affinity to a radical polymerizable monomer, thereby imparting high mechanical strength, flexibility and durability when used for a medical and/or dental curable composition, and an inorganic filler surface-treated with the novel silane coupling agent and a novel medical and/or dental curable composition. A silane coupling agent including repeating units such as a urethane bond and polyethylene glycol (ether bond) at a specific position is used.
-
Diamine Compound Having Phosphorylcholine Group, Polymer Thereof, and Process for Producing the Polymer申请人:Nagase Yu公开号:US20100036081A1公开(公告)日:2010-02-11Highly polymerizable diamine compounds having a phosphorylcholine group are disclosed. High-molecular weight polymers are obtained from the highly polymerizable diamine compound having a phosphorylcholine group as a monomer, and the polymers have improved mechanical strength, water resistance and heat resistance while maintaining excellent biocompatibility and processability of MPC polymers. Processes for producing the polymers are disclosed. The diamine compounds having a phosphorylcholine group are represented by Formula (I). The polymers contain at least 1 mol % of a specific structural unit with a phosphorylcholine group represented by Formula (II) and have a number average molecular weight of not less than 5,000. In the processes, the diamine compound is used as a monomer.
-
[EN] OXIME ESTER PHOTOINITIATORS<br/>[FR] PHOTO-INITIATEURS À BASE D'ESTER D'OXIME申请人:BASF SE公开号:WO2021175855A1公开(公告)日:2021-09-10Disclosed are α-oxo oxime ester compounds based on carbazole derivatives which have specific substituent groups useful as a photoinitiator, as well as photopolymerizable compositions comprising said photoinitiator and ethylenically unsaturated compounds. The photopolymerizable compositions are useful, for example, in photoresist formulations for display applications, e.g. liquid crystal display (LCD), organic light emitting diode (OLED) and touch panel.
-
Bishydroxyureas申请人:3M Innovative Properties Company公开号:US06121323A1公开(公告)日:2000-09-19Bishydroxyureas are provided that inhibit the enzyme 5-lipoxygenase. These compounds have the formula I ##STR1## wherein R.sub.1, R.sub.2, R.sub.3, R.sub.4, A and M are defined herein. Also disclosed are pharmaceutical compositions containing such compounds and methods of inhibiting the enzyme 5-lipoxygenase using such compounds.
-
SULFAMIDES AS TRPM8 MODULATORS申请人:Matthews Jay M.公开号:US20100160337A1公开(公告)日:2010-06-24Disclosed are compounds, compositions and methods for treating various diseases, syndromes, conditions and disorders, including pain. Such compounds are represented by Formula I as follows: wherein Y, R 1 , R 2 , R 3 , R 4 , R A , and R B are defined herein.揭示了用于治疗各种疾病、综合症、症状和障碍的化合物、组合物和方法,包括疼痛。这些化合物由以下的化学式I表示: 其中Y,R1,R2,R3,R4,RA和RB在此处被定义。
表征谱图
-
氢谱1HNMR
-
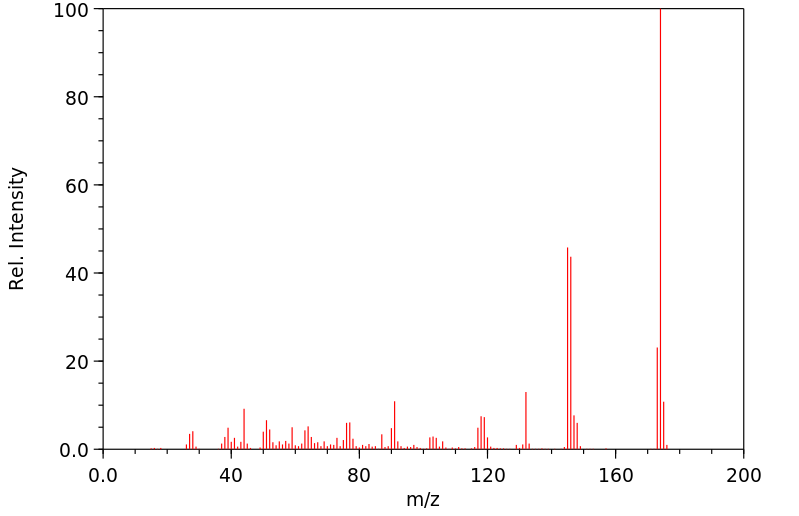
质谱MS
-
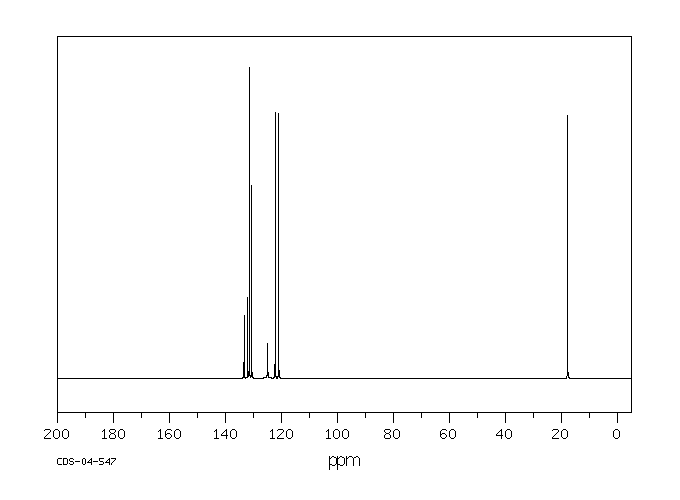
碳谱13CNMR
-
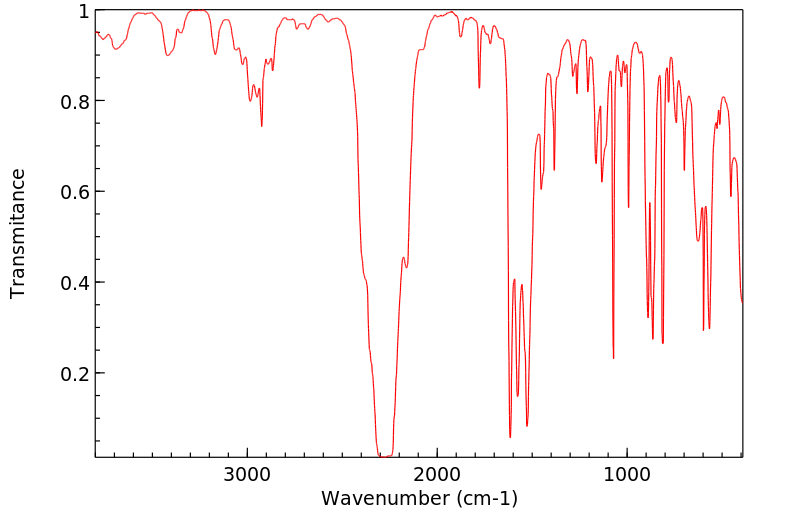
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息